
Covance Inc. and Pathoquest have announced a collaboration to provide next-generation sequencing (NGS) based biosafety assessments to detect and identify viral contaminants within biologic compounds.

Covance Inc. and Pathoquest have announced a collaboration to provide next-generation sequencing (NGS) based biosafety assessments to detect and identify viral contaminants within biologic compounds.

Intertek is now offering comprehensive services for the development of orally inhaled and intranasal drug products (OINDPs) for both small molecules and biologics.
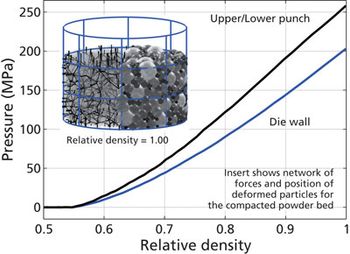
Screening methods and predictive models address tenacious tablet-sticking problems.

Progress in delivery science, manufacturing technologies, and commercialization are playing critical roles in advancing the development of complex parenteral drug formulations for new drug substances having a variety of formulation challenges

Generic Drug Super Office Takes Shape

Bend Research adds powder-filling technology.

The benefits of solvent-free processes and techniques specifically optimized for coating new dosage forms such as orally disintegrating granules are reviewed.

GSK and the Gates Foundation will invest a combined $1.8 million in early stage research into vaccine thermostability.

In this episode, we?ll discuss how an aging global population and the need for continual innovation in the pharmaceutical industry factor into drug delivery systems that move beyond traditional pills and capsules into transdermal drug delivery.

Pliva?s new Oral Solid Forms Facility will increase Pliva's production capacities for tablets and capsules.

Capsule design was developed for patients who have difficulty swallowing.

Interview of Detlev Haack, head of R&D, Hermes Pharma on the characteristics, benefits and manufacturing Methods of effervescent tablets

Stephen Tindal, Director Softgel Formulation at Catalent, talks with Pharm Tech about manufacturing issues with soft gels.

Kansas State University to build Bulk Solids Innovation Center in Salina, Kansas.

Governor Jerry Brown cites physician notification requirements for dispensing biosimilars as premature in the absence of FDA specifications for interchangeability.

Scientific Digital Imaging's Synbiosis Division introduces SynStats statistical analysis software.
HERMES PHARMA has pioneered user-friendly dosage forms. Our effervescent and chewable tablets, lozenges, orally disintegrating granules and instant drinks are convenient to use, easy to swallow and taste good.

Aesica expands aseptic capabilities at its Nottingham, UK site with the acquisition of new pre-filled syringes manufacturing equipment.

Pfenex modifies its contract with US government for Anthrax vaccine.

3M Drug Delivery will manufacture transdermal patch for ProStrakan.

Advanced Biosciences Laboratories will support the development and production of a novel Shigella vaccine candidate for PATH.

Industry experts discuss the importance of characterization studies during biosimilars development and related analytical methods.

Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.

An industry leader since 1933, Catalent develops and manufactures over 80% of the world's Rx softgel products with 200+ products on the market in 80+ countries.

Boehringer Ingelheim initiates a recall due to the potential for extrinsic foreign particles in the API used to manufacture Spiriva Handihaler (tiotropium bromide inhalation powder) capsules.