
Models and modeling software gain a foothold in solid-dosage manufacturing process design.

Models and modeling software gain a foothold in solid-dosage manufacturing process design.

Crushing, fracturing, and bending tests quantify hardness.
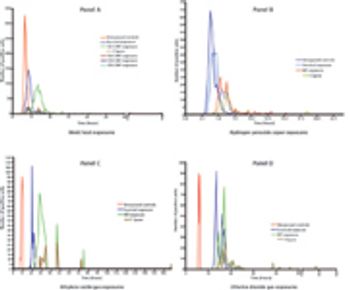
The authors report on the range and distribution of grow-out times for biological indicators exposed to sublethal sterilization processes.

GSK expands its vaccines platform technology expertise by acquiring Okairos, a Swiss-based company.

The South Wales, UK contained-manufacturing operation was built in less than a year using design-to-manufacture and modular approaches.

Adoption of single-use systems and more flexible systems drive innovation.

Greater automation and the adoption of solid versus perforated pans are some recent advances.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Crushing, fracturing, and bending tests quantify hardness.

Thomson Reuters released a report that provides a view of the challenges facing companies entering the United States biosimilar drug market.

Drug manufacturers today are increasingly challenged to find new, effective methods for the delivery of active pharmaceutical ingredients, and many are discovering that orally disintegrating technologies can help.
Advances in monoclonal antibody separation technologies are largely driven by the need to increase efficiencies and reduce cost.

New platform technologies and polymer chemistries may facilitate self-administration, longer-term delivery, and targeted delivery of parenteral drugs.

The Global Vaccine Action plan maps out a strategy to increase access and R&D for vaccines.

Podcast interview with Nick Johnson, Strategic Marketing Director for Modified Release Technologies at Catalent Pharma Solutions

Applications of ZFN technology in biopharmaceutical cell-line engineering.

The rejection by India's Supreme Court on Novartis' Glivec/Gleevec (imatinib mesylate) and other recent case law raise important issues on patent strategies for solid forms.

Solid-state chemistry is an important part of drug development, and public research is advancing the field.
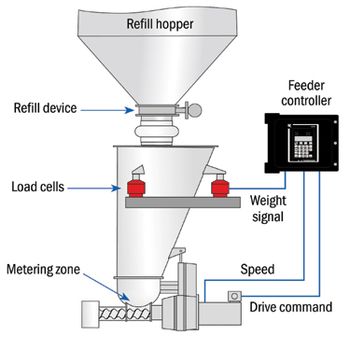
Loss-in-weight feeders provide high accuracy for batch or continuous processes.
Parenteral drug delivery offers a variety of challenges but also opportunities. The author examines recent developments in nanotechnology-based drug delivery and other advances in injection-based drug delivery.
Despite overall manufacturing rationalization in the pharmaceutical/biopharmaceutical industry, the large pharmaceutical companies continue to invest in biologic-based manufacturing. The author provides an update in recent investment activity among the pharmaceutical majors in biologic-drug substance, vaccine, and parenteral drug manufacturing.
The authors describe a holistic and integrated approach to focus on the linkage of the prefilled syringe with the four phases of product design, development, operation, and control.

Researchers recently developed a drug-delivery system to mitigate problems associated with jet-injection drug delivery, and also improved on the design and operation of microscale actuators as a possible drug-delivery method.

Results of an American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable survey found that there is real interest in continuous processing and the use of flow chemistry, but hurdles remain.

Metrics Inc. offers Cleantaste technology, which enables polymer coating of individual drug crystals to produce particles sized 25 um to 125 um.