
Sanofi and POZEN sign a license agreement for the commercialization of omeprazole and aspirin combination product.

Sanofi and POZEN sign a license agreement for the commercialization of omeprazole and aspirin combination product.

Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

Merck & Co gets FDA approval to manufacture bulk varicella at its in Durham, North Carolina for use in chickenpox and shingles vaccines.

Baxter and Coherus Biosciences colaborate to develop and commercialize a biosimilar version of etanercept for Europe, Canada, and, Brazil.
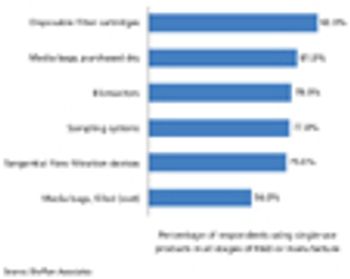
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Prequalification of Sanofi Pasteur?s Menomune vaccine makes it eligible for purchase by United Nations agencies.

Avacta?s Optim 2 instrument provides a practical solution for determining the conformational stability of adjuvant bound vaccines, eliminating the need for awkward and time consuming standard analytical techniques. [more?..]

The advantages of using an automated powder dispensing system in a ventilated balance enclosure for efficient handling and effective containment of potent compounds are discussed.

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.

The Bosspak VTC 100 electronic tablet and capsule counter from Romaco?s is designed to fill pharmaceutical solids or food supplements into bottles at high speed.
Case studies on the manufacture of a bluk powder and the development of a tablet show the application of QbD principles.

A Sanofi Pasteur flu-vaccine trial in adults 65 years of age and older meets a primary endpoint for superior efficacy.

FDA's draft guidance provides answers to questions received on FDA's abbreviated new drug application stability guidance.

Conventional cell-imaging systems that provide high quality data can be expensive and complex to use. New systems from Biotek Instruments are designed to overcome these issues.

Installation of a quaternary chiral center with high enantioselectivity using memory of chirality enabled the six-step synthesis of the desired active compound.

Direct tablet compression is simpler than wet or dry granulation, but obtaining the right flow properties to ensure good compaction and uniform drug distribution can be a challenge. Porous silica gel, when used as a glidant, can address these issues.

Extensive adoption of single-use technologies and unidirectional flow reduces cross-contamination risk in the company?s new biomanufacturing facility.

Conventional cell-imaging systems that provide high quality data can be very expensive and complex to use. New systems from Biotek Instruments are designed to overcome these issues.

Reversible nanoencapsulation technology may make it possible to deliver high molecular weight drugs directly to target cancer cells, increasing efficacy and reducing side effects.

Experts, including researchers at I Holland, Natoli, and Rutgers University’s Engineering Research Center for Structured Organic Particulate Systems, are seeking a greater understanding of the fundamental causes of tablet sticking and are developing predictive models to more quickly find solutions to specific sticking problems.

RABS is a flexible barrier system that maximizes product control but minimizes operator interaction when best practices are followed.

Rapid screening of critical API properties can quickly identify the best approach for increasing bioavailability.

The companies initially focused on five biosimilar products.

Adamis Pharmaceuticals agrees to license and potentially acquire 3M's Taper Dry Powder Inhaler technology.

Pfizer, Merck, Sanofi, and AstraZeneca are among the companies reporting revenue declines from generic-drug incursion. A look at what the companies are doing to stimulate growth.