
The authors study the effects of sweet potato and cocoyam starches on the compressional and mechanical characteristics of a paracetamol tablet formulation using cornstarch BP.

The authors study the effects of sweet potato and cocoyam starches on the compressional and mechanical characteristics of a paracetamol tablet formulation using cornstarch BP.

Innovators and generics makers are staking out positions on follow-on proteins and other manufacturing issues.

X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage. This article describes the basic principles of the technique and provides examples of its potential applications.

"Stable Liquid" Technology Permits Heptavalent Vaccine Against Botulism

UK MHRA Okays Chiron Flu Vaccine Plant; FDA Approval Still to Come

Big Pharma Companies Team Up to Develop Once-Daily, Triple-Combination HIV Drug

This article outlines a comprehensive approach for organizing a firm's aseptic operations, including planning for routine and nonroutine interventions, establishing effective process simulations, and determining which vials to incubate.

New fixed-dose combination drugs aim to enhance safety and efficacy, while regulators clear a path for more drug–device combination products.

The author points out some major obstacles to effective scale-up and describes methods available to pharmaceutical scientists for addressing scalability issues.

In the manufacture of many pharmaceutical products, dry-particle blending is a critical step that directly affects content uniformity.

Novartis to Expand Generics Hold

CBER Draft Guidance for Spore-Formers and Plasmid Vaccines

Extrusion-spheronization and pellet compression are effective means of developing first-order kinetic, controlled-release drug delivery systems of azithromycin (AZI). The authors prepared, evaluated, and optimized AZI formulations and assessed the stability of the selected formulation under accelerated storage conditions.

Pharmaceutical Science & Technology Innovations

Laser-Marking Technique Improves Tablet Branding

ISA-88 Automation Standard Gains IEC PAS Status

Albumin-Bound Nanoparticle Drug Nabs FDA Approval

MHRA to Reinspect Chiron Flu Vaccine Plant

Engineered Oilbodies Produce Vaccines and Adjuvants

Engineered Oilbodies Produce Vaccines and Adjuvants

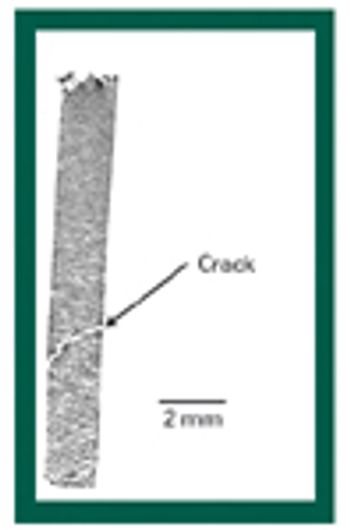
The author reviews the effects of moisture on flow properties, tensile strength, Heckel plot, energies involved in compaction, and elastic recovery.

From solving complex formulation challenges to delivering full product-development programs, outsourcing services organizations provide valuable expertise, experience, and technical know-how.

The authors examine the effects of three superdisintegrants on the dissolution and absorption of tenoxicam from solid-dispersion formulations.

Microspheres of Eudragit RL were developed for colonic delivery of albendazole. The effects of polymer concentration, stirring rate, and concentration of emulsifier on particle size and drug loading were studied. A comparative in vitro drug release study of the optimized formulation was carried out.