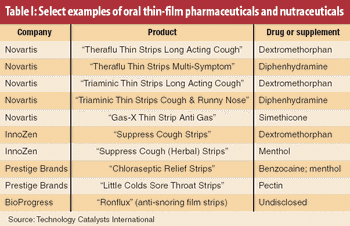
Using a novel automated microfilling system, the authors demonstrate that roller compaction followed by milling is a viable preprocessing technique for high-dose chemical-in-capsule dosage forms. The process results in higher bulk and tapped densities for drug substances compared with milling alone.