
FTC Plans to Study Competitive Impacts of Authorized Generic Drugs

FTC Plans to Study Competitive Impacts of Authorized Generic Drugs

Reverse Genetics Avian Influenza Vaccine Approved in France

GSK Begins Clinical Trials of Two H5N1 Vaccines
Cell-culture technology and financial incentives give influenza vaccine makers a much-needed shot in the arm, but many downstream processing issues remain unaddressed.

Creation and qualification of scale-down models are essential for performing several critical activities that support process validation and commercial manufacturing. As shown in Figure 1, these activities include process characterization and production support studies that are performed to evaluate column and membrane lifetimes, demonstrate clearance of host-cell impurities and viruses and troubleshoot manufacturing issues. While the underlying fundamentals are relatively the same as those when scaling up, some unique considerations should be taken when scaling unit operations down.4

Once the brightest students are interested in bioprocessing, it is vital that they are prepared and inducted into industry.

Progress Continues in Vaccine and Antiviral Development

BMS, Sanofi-Aventis Settle Over Generic Plavix

Implementing a PAT Strategy

Chiron Withdraws Measles Vaccine

GSK Loses Bid to Block Generic "Flonase"

Development Begins on Mutated H5N1 Virus Vaccine

Novavax, Bharat Biotech Team for Pandemic Influenza Vaccine Development
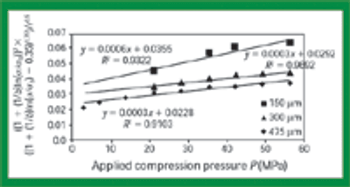
Quantitative data from the literature show strong relationships among average particle size, powder densification, tensile strength, and hardness.
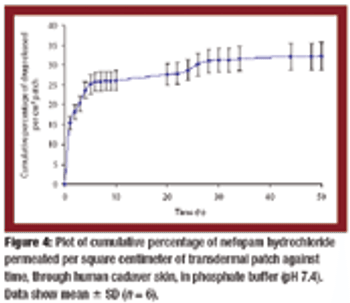
Transdermal matrix-type patches of Nefopam hydrochloride with a combination of pressure-sensitive adhesives were developed. The polymeric composition provided a controlled and sustained release of the drug from the patches and demonstrated favorable physicochemical characteristics.

BMS Receives FDA Approval for Transdermal Antidepressant
Because of the growing popularity of single-use materials, the identification, characterization, and qualification of new materials used for disposable processes have become increasingly important for both regulatory and production purposes. This article describes one approach to identifying and validating the materials used in a disposable filling process.

GSK Gains Approval for Rotavirus Vaccine in Infants; FDA Review Extended for Merck’s Shingles Vaccine

Human plasma provides a rich source of therapeutic medicines, including gamma globulins, coagulation factors, albumin, alpha anti-trypsin and others. In 2001, sales of immuno gamma-globulin (IgG) were estimated at $2 billion with a production rate of 50 metric tons for the year.1 A number of therapeutic products have been introduced including Gammimune from Bayer, RhoPhylac from ZLB Behring and Octagam from Octapharma.

FDA Recommends Changes for 2006-2007 Flu Vaccine

Baxter Wins Contract to Develop Cell-Based H5N1 Vaccine

Cambrex (East Rutherford, NJ) to produce Geron (Menlo Park, CA) telomerase anti-cancer vaccine. Generex (Toronto, ON.) files IND for synthetic vaccine to stimulate cell-mediated immunity to avian influenza. Dow Agrosciences (Indianapolis, IN) wins USDA approval for veterinary vaccine, the first manufactured via plant cell culture. G-8 nations pledge billons for vaccine production.

Merck wins approval for rotavirus vaccine as Sanofi ships investigational H5N1 vaccine to NIH and CDC explores new flue diagnostics and novel vaccine-production technology.

Dow AgroSciences Receives Regulatory Approval for Plant-Made Vaccine
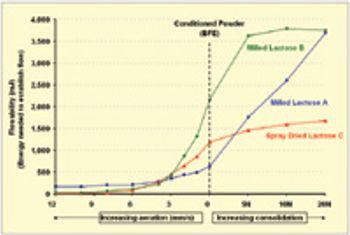
The pharmaceutical industry's focus on process understanding, monitoring, and control is driving manufacturers to take greater steps toward identifying possible manufacturing bottlenecks earlier in the development process. For tablet, capsule, and excipient producers, such efforts include taking a closer look at the flow-ability of their powders.