Drug Development
Latest News
Latest Videos

More News

Webinar Date/Time: Fri, Dec 13, 2024 11:00 AM EST

CHMP has granted Eisai Europe and Biogen a positive opinion for the use of their monoclonal antibody therapy in treating early Alzheimer’s disease.

Avantor's newly launched expanded Innovation Center in Bridgewater, NJ, is designed for integrated workflows and features purpose-built collaboration spaces.

Executives at Avantor talk about the future of the biopharmaceutical industry and the anticipated impact that a wave of next-generation biotherapeutics will bring.

Pharmaceutical Technology® caught up with Brittany Hayes, PhD, Highly Potent & Oncology Platform Director at CordenPharma, after AAPS PharmSci 360 to talk about the challenges and quality requirements involved in the development and manufacture of peptide-based oral dosage forms.

Webinar Date/Time: Wed, Dec 4, 2024 11:00 AM EST

Webinar Date/Time: Tue, Dec 3, 2024 11:00 AM EST

The new Institute for Cell Therapy Discovery and Innovation will utilize expertise to develop cell therapies for cancer, autoimmune diseases, and infections.

The companies will work to create climate-friendly pMDIs using Orbia’s low global warming potential propellant.

In the collaboration, the new company, Oblenio Bio, has been granted an exclusive option to license LBL-051, a tri-specific T-cell engager antibody, from Leads Biolabs.

A genetic medicines customer has selected the company’s binders for commercial development of the customer’s product.
Automation, miniaturization, and new software algorithms are improving throughput and accuracy.

Synaffix, a Lonza company, has licensed its ADC technology to BigHat Biosciences to be combined with the latter's ML design platform to develop new ADCs

CDER Director Patrizia Cavazzoni and CBER Director Peter Marks provided an update on the rare disease innovation hub in a new FDA Voices blog post.

The partnership will use ViaNautis’ proprietary polyNaut technology platform to develop genetic medicines that can precisely target tissue types.

Webinar Date/Time: Fri, Nov 22, 2024 11:00 AM EST

The collaboration aims to advance clinical development of a Phase I drug candidate, MRT-6160, and to explore other therapeutic opportunities across multiple indications.

POLYOX™ Polyethylene Oxide Essentials: An Expert Guide to Polymer Properties and Drug Formulation
Webinar Date/Time: Tue, Nov 19, 2024 11:00 AM EST

The laboratory is on the same site as BioDuro-Sundia’s new Compound Management Center, which was launched earlier in October 2024.

Merck is acquiring the Yale-spinout, which develops direct DNA modification-enabled cancer treatments.
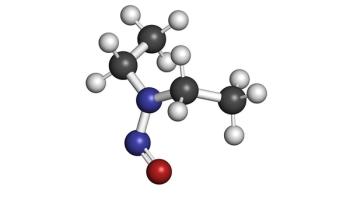
This article presents an overview of FDA’s recent update to the guidance document, Control of Nitrosamine Impurities in Human Drugs.

The Cambridge-GSK Translational Immunology Collaboration builds on an existing scientific relationship between the biopharma company and the university, with the aim of improving outcomes for patients.

At AAPS PharmSci 360, Karunakar Sukuru, RPh, PhD, the global vice-president-Rx Product Development, Pharma and Consumer Health, at Catalent Pharma Solutions, discussed lipid-based formulations and tackling bioavailability challenges for oral biologics.

Karunakar Sukuru, RPh, PhD, the global vice-president-Rx Product Development, Pharma and Consumer Health, at Catalent Pharma Solutions, spoke on recent challenges and trends in softgel formulations.
Bala Addepalli, PhD, Director, R&D New Modalities Portfolio, Waters Corporation, discussed trends in new analytical tools for RNA characterization and gave highlights of his talk at AAPS PharmSci 360.