Drug Development
Latest News
Latest Videos
More News

Poor API quality may often lead to delays in production and a shortage of supply.

Scientific, economic, and practical factors should be considered when choosing between the frozen state and lyophilization.

BioPharm International® spoke with Lun Xin, associate director at WuXi Biologics, about high-concentration biologics and some of the biggest challenges associated with their formulation.

The PharmTech Group interviewed Edwin Stone, CEO of Cellular Origins, about how technological advances will impact the biopharma industry in the future.

BioPharm International® sat down with Noah Kopcho, field application scientist at Gyros Protein Technologies, to talk about the challenges in the production of AAV vectors.

Incorporating sustainable practices into process designs as early as possible ensures optimal performance.

Aviva Capital Partners and developer Socius are investing £1 billion to develop a cancer research and treatment center in Sutton, London.

The pharma industry is focused on strengthening its foundation, embracing innovation, and future-proofing its path forward.

Bempikibart (ADX-914) is a human anti-IL-7Rα antibody that blocks the IL-7 and TSLP pathways, which have been implicated in driving T cell-mediated pathological processes in autoimmune diseases.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about the impact geopolitical changes in Europe might have on the bio/pharmaceutical industry.
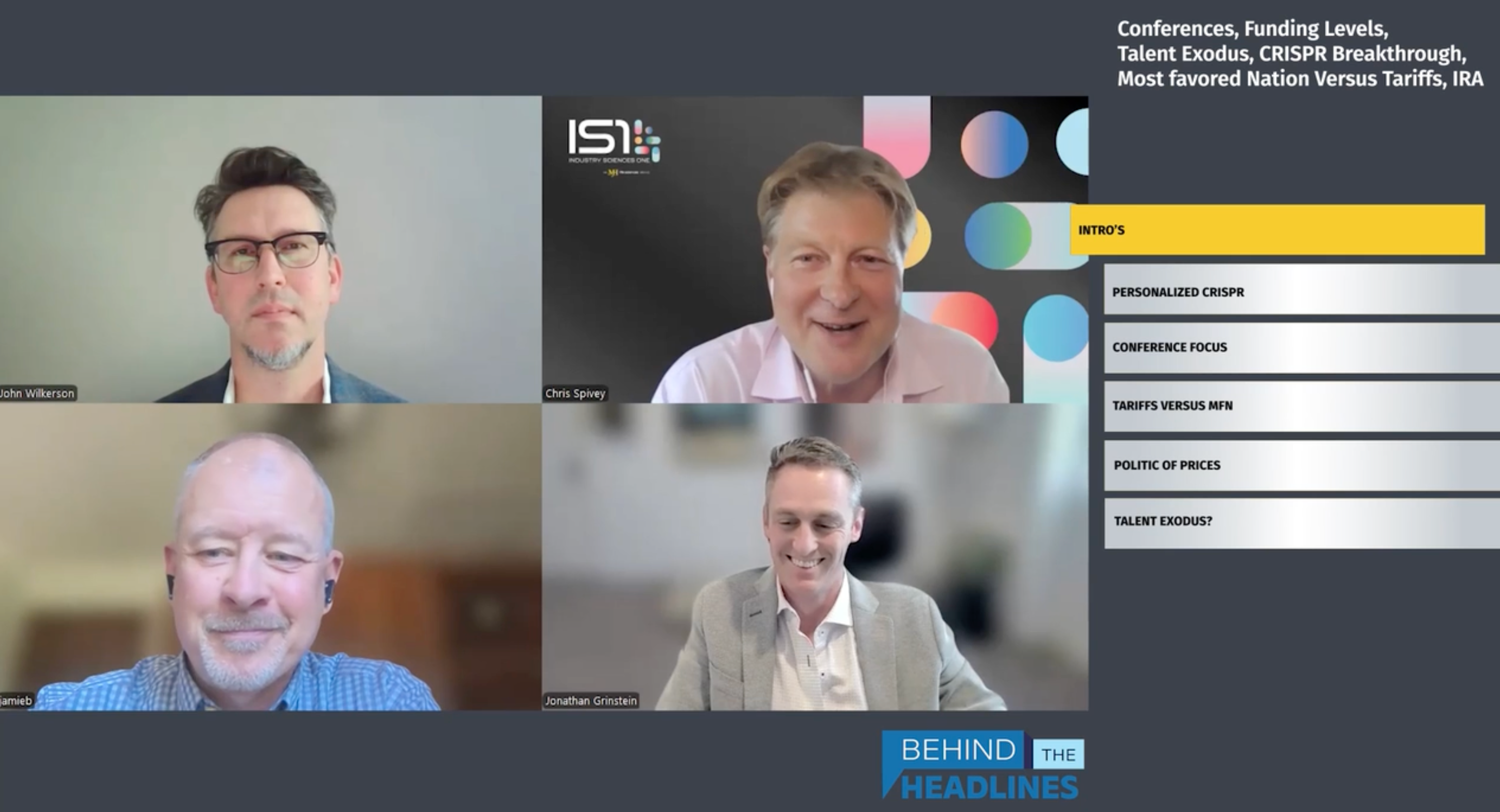
Donald Ingber, MD, PhD; Miguel Forte, MD, PhD; Ali Pashazadeh, MRCS, MBA; and Adam Inche, PhD, MBA go behind the headlines to examine the fast-changing landscape of manufacturing re-shoring, tariffs, NIH budget cuts, and the outlook for cell and gene therapies.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about how the political landscape may impact the pharmaceutical industry.

The AbYlink technology enables a seamless, reproducible conjugation of payloads to antibodies or ADCs.

Under the partnership, Pharmaron will validate and promote the application of CN Bio’s PhysioMimix technology and will integrate OOC technologies into its R&D platform.


The project, the North African Dementia Registry, will unite multiple entities for the purpose of developing a comprehensive dataset to advance the research community’s understanding of Alzheimer’s disease and other dementias in diverse populations.

Pharmaceutical Technology® Group spoke with Erik Wiklund, CEO of Circio, about the future of M&A in the bio/pharmaceutical industry and the struggle small biotech companies face raising funds.

The commitment will include new, state-of-the-art R&D facilities as well as new or expanded manufacturing sites in multiple states.

The PharmTech Group interviewed Edwin Stone, CEO of Cellular Origins, about the impact of the changing political landscape on the pharmaceutical industry.
Mike Exton, Andrew Thomson, Konstantinos Tsioris, and Cheryl Barton go behind the headlines to discuss the advancement of neuroscience, weight-loss drugs, and the evolving political climate’s impact on the industry.

Vitrakvi (larotrectinib) was first granted accelerated approval by FDA in November 2018.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about the use of artificial intelligence and machine learning in pharmaceutical development and manufacturing.

The company has plans to invest $23 billion during the next five years to expand manufacturing and R&D in the US, which will include seven new facilities.

The PharmTech Group interviewed Edwin Stone, CEO of Cellular Origins, about the bio/pharmaceutical trends from 2024 that continue to impact the industry in 2025.

Lack of skills in the AI realm was a distant second among those surveyed about the biggest barrier to innovation while using the technology.