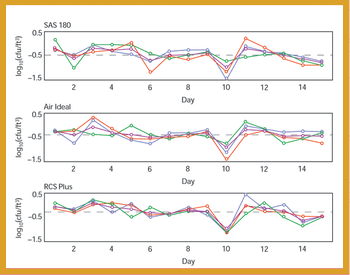
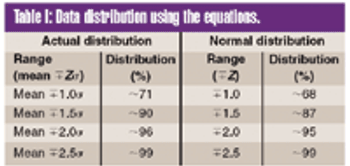
The author proposes a new analytical graphic, the sector chart, which presents data that cannot be adequately presented with current graphs.This chart combines features of zone charts with the basic principle of precontrol charts. It addresses engineering control and is superior for representing data such as beneficial and adverse trends.