
Researchers are improving photodynamic therapies with new photosensitizers and light sources for deeper tissue penetration, more site-specific treatments, and reduced side effects.

Researchers are improving photodynamic therapies with new photosensitizers and light sources for deeper tissue penetration, more site-specific treatments, and reduced side effects.

Pharmaceutical Science & Technology News

On the other hand, if we don't keep up the pressure on counterfeiting in the US, we may share the plight of the developing world, where nearly 25% of all drugs sold are fakes.

Defenders of the research-based industry are hoping for an early Christmas present from the European Court of Justice (ECJ). All the signs are that a small but significant victory is on the way against parallel importing. Right at the end of October, a senior judge responsible for a leading case at the court made clear his view that international drug firms do not necessarily have to make life easy for parallel importers. The formal ruling on the case at issue is expected within weeks.
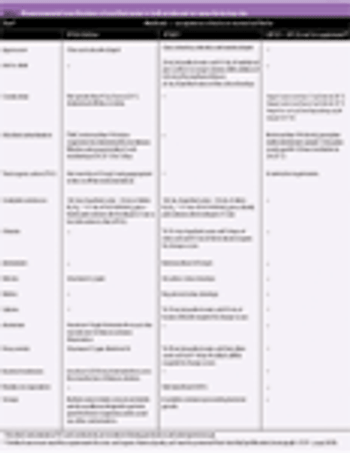
A harmonized global specification is possible providing that the procedures and acceptance criteria defined are acceptable to regulatory authorities in all regions.


Amid the finger-pointing, hand-wringing, and bloviating, the supply of hard, technical information remains frustratingly small.

A discussion of the validation and operation of two commercially available vapor-phase hydrogen peroxide decontamination systems is presented, based on a hands-on examination of both systems.

With creative engineering and sophisticated chemistry, industry scientists offer big solutions for attaining small particle sizes and narrow distributions.

Phase II of FDA's GMP modernization plan may involve revising regulations to fit new policies and inspection and review programs.
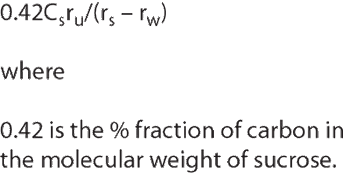
The implementation of a total organic carbon (TOC) method into the United States Pharmacopeia (USP) has its origins in the early 1990s, when the Water Quality Committee (WQC) of the Pharmaceutical Manufacturers Association (PMA, later renamed PhRMA) debated improvements in the testing of purified water (PW) and water-for-injection (WFI). The resulting inclusion of modern analytical techniques replaced much older methods - some of which had been listed in the USP for more than 150 years. Finally, two new regulations were put in place: Chapter for conductivity, which replaced a series of individual ion tests; and Chapter which replaced the oxidizable substances test with a TOC method.

Information retrieval (IR) deals the representation, storage, organization of unstructured applying natural-language processing,semantic relationships, linguistic analyses,behavioral histories, and “fuzzy” statistical techniques to help human quickly find and retrieve the information they seek.

This article describes a novel approach to a scale-up management system, based on a holistic view of the scale-up lifecycle and an accompanying electronic development record of the information created.

The author argues that information technology solutions will play a major part in confirming-by experimental and statistical validation-that bioprocesses may be interchangeable and therefore generic. The bioprocessing ANDA will define both in-house and contract manufacturing.

Pharmaceutical science and technology news

Pharmaceutical science and technology news

Freeze pelletization is a new and simple technique for producing spherical pellets for pharmaceutical use.

The authors review current industry practices and regulatory expectations for the aseptic processing of sterile drugs. They compare and outline critical issues in current manufacturing technology and capabilities with regulatory requirements.

Using dimensionless analytical models, the authors establish the relationships among the essential parameters of a kneading complexation process.

FDA and industry discuss proposals for regulating combination diagnostics, medical devices, and pharmacogenomic-based drug products.

By enhancing stability and in vitro drug-release characteristics, limonene enantiomers are a natural-source alternative for developing and characterizing self-nanoemulsified drug delivery systems.

Big Pharma is ramping up capital spending in parenteral manufacturing at the same time that contract manufacturers are completing their own major investment programs.

Transdermal drug technology specialists are meeting demands for methods that can painlessly deliver larger molecules in therapeutic quantities.

Steaming-in-place (SIP) is a widely adopted method for the in-line sterilization of processing equipment. The main advantage of SIP relies on manipulation reduction and aseptic connections that might compromise the integrity of the downstream equipment.

Single-use products enhance sterile filtration by making it easier to maintain sterility and reduce cross-contamination risks.