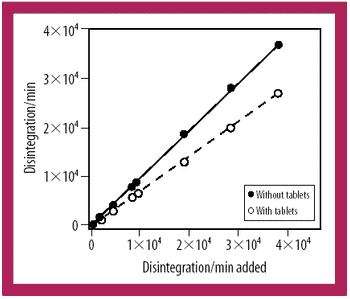
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.

A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.
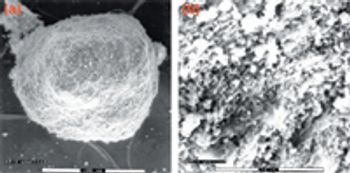
Although agitation improves drying efficiency and ensures uniformity of the final dry material, it can also affect the physical properties of the product as it dries. This study evaluates the effect of scale up and equipment selection on an active ingredient undergoing granulation during the drying process.

Chemical purity is the most important quality characteristic of a pharmaceutical substance. This article describes the latest scientific and technological advances to meet recent pharmacopoeial and regulatory requirements regarding the control of organic impurities in synthetically produced active substances. Future developments and suggestions for those working in quality control and raw material selection are discussed.

A polymer excipient that reportedly reduces spray time has received its first registration in a finished drug product.

Eli Lilly and Alkermes’ rival formulation to Pfizer’s inhaled insulin, Exubera, has cleared a Phase II clinical trial.

The global pharmaceutical industry is currently in a state of flux according to Frost & Sullivan.

Warning Letter: Similasan

Poly(ethylene oxide) is gaining the attention of research and development organizations and its application is extending into a wide range of drug delivery systems.
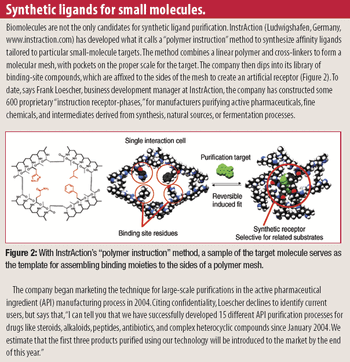
Rapidly increasing cell-culture yields have thrown an increasing burden on downstream processes just as price pressures are pushing process developers to look for economies in every purification protocol. The time-honored, effective, and expensive war-horse, Protein A, is beginning to feel some competition from small-molecule mimetics.

The European Commission has released its second report to the Council and European Parliament addressing the developments and implications in patent law concerning biotechnology and genetic engineering. The study, which centres on the patentability of inventions relating to stem cells, concluded that those capable of developing into human beings, totipotent stem cells, are to be excluded from patentability on the Directives' grounds of human dignity.

IAdvances in pressurized metered-dose inhalers (pMDIs) in terms of formulation capability and the performance of the container closure system enable products to be developed faster and with less technical risk. Despite new delivery devices for new molecules breaking into the pMDI market, pMDIs have the ability to gain regulatory approval significantly faster than a novel device, which could save a company many hundreds of millions of pounds.
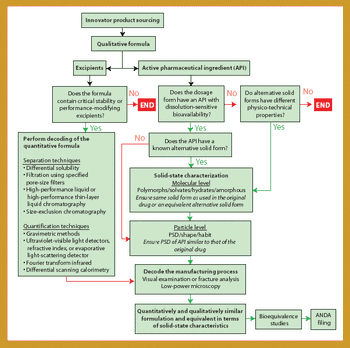
Being the first to gain the most is a fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. For a generics company to maintain revenue growth in a market in which product prices continue to fall, it must secure a continuous flow of new products, with quality and speed to market being key drivers. Thus, generics companies must be highly skilled in product and process development (1), the generics business, and achieving bioequivalence-the most critical development area.

The question of pharmaceutical pricing is going to be resurrected by the European Commission, despite French President Jacques Chirac's rejection of the new EU treaty. Günther Verheugen, the Commission vice president, made this announcement at the annual meeting of the European Federation of Pharmaceutical Industries and Associates in Brussels (Belgium) responsible for competitiveness.

By forming strategic collaborative partnerships with contract research organizations, pharmaceutical companies can take advantage of several strategies for accelerating drug development, maximizing profitability, and extending patent exclusivity.
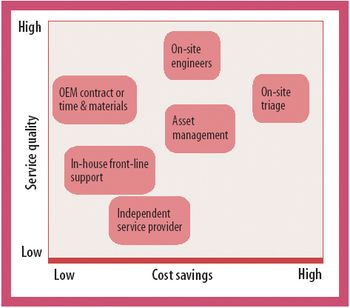
New maintenance models focused on efficiency improvements can deliver cost savings and enhanced service quality.

Reading the good automated manufacturing practice (GAMP 4) guide acquaints you with the now classic and almost famous V-model.1 The V-model, originally used for describing a validation workflow of IT and automated systems, is easy to understand and very good at ensuring that the requirements and design are built into the final solution. It is also extremely versatile and can be used for almost any type of validation task you could meet in a development phase.

The aim of this study was to analyse the process of tablet formation and the properties of the final tablets for six different carrageenans. The carrageenans used were based on the basic types of ?-, ?- and ?-carrageenan. Microcrystalline cellulose was used for comparison. Determination of material properties, compression analysis and tablet properties were described. Water content, particle size and morphology, glass transition temperature, and crystallinity were studied. The results show that the carrageenans are predominantly amorphous fibres, which are in the rubbery state during tabletting.


Able Interim Chief Resigns Amid Drug Recall

First Contraceptive Spray Offers Advantages over the Pill

Police and protests at BIO 2005.
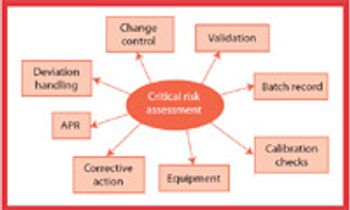
A stepwise, process risk-assessment approach can facilitate the identification and understanding of critical process parameters, quality attributes, and in-process controls. This approach can lead to more use of science- and risk-based regulatory practices to simplify the regulatory requirements for changes to synthetic processes and to support the underlying quality systems that ensure compliance.

Can macromolecular processes learn from small-molecule experience? Burdened by exploding bioreactor productivity, architects of downstream bioseparation technology are looking into the drug industry's past for inspiration, while small-molecule companies adopt techniques pioneered by biotechnology. (The first of three articles on the current state of separations.)

Genentech Anticipates Full-Capacity Manufacturing

Molding Technique Improves Nanoparticle Production