
Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

A bipartisan bill that would establish a regulatory pathway for the approval of biosimilars was introduced into the US House of Representatives last week.

The US Pharmacopeial Convention (USP) and the National Institute for the Control of Pharmaceutical Biological Products (NICPBP), China's agency for overseeing the quality of large- and small-molecule drugs, signed a memorandum of understanding (MOU) to bolster the quality of medicines in China and in the countries that buy Chinese drug products, including the United States.

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...

Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

The Synthetic Organic Chemical Manufacturers Association (SOCMA) said last week that Congress is likely to the include inherently safe technology (IST) measures in proposed chemical site-security legislation that is likely to be introduced in late winter or early spring.

Weakening fundamentals in 2008 and projected for 2009 dampen the outlook for pharmaceutical chemical outsourcing, but near-term growth and investments levels in R&D and capital spending remain fairly robust.

Gilles Cottier, SAFC's new president, plans to continue a growth strategy that emphasizes value-added technologies positions, growth in Asia, and customization in its supply-chain solutions.

FDA issued a notice in the Federal Register last week requesting comments on the automated collection of four additional pieces of information that are not available in the US Customs and Border Protection's (CBP) data set.

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...
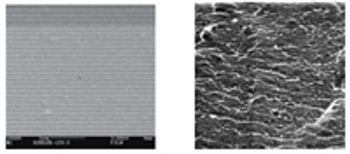
The authors investigate the effects of a polyethylene glycol plasticizer and water on cellulose acetate film properties.

Industry has changed, but its basic tenets have not. INTERPHEX's RJ Palermo discusses a 7-step process to keep pharma moving forward.

Brief pharmaceutical news items for March 2009.

Contract manufacturers of APIs and intermediates report gains, but express caution.

Contract manufacturers deploy a business model using operations in the US and Western Europe with facilities in Asia.

Also, GPC Biotech AG and Agennix to merge; BASi appoints COO of scientific services; more...

The United States Trade Representative (USTR) is seeking documentation from chemical companies to identify possible non-tariff trade barriers, created by the European Union's Registration, Evaluation, Authorization and restriction of Chemicals (REACH) regulation, which would be inconsistent with the EU international trade obligations under World Trade Organization (WTO) rules, according to an informational release by the Synthetic Organic Chemical Manufacturers Association (SOCMA).

After two years of hearings, more than 5000 pages of expert testimonies, and 939 medical articles, a special federal court ruled that there was little, if any, evidence to support the claim that substances in the measles, mumps, and rubella vaccine (including the use of thimerosal) had led to the autism of three children.

Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

The US Food and Drug Administration sent letters to manufacturers of certain opioid drug products, indicating that these drugs will be required to have a Risk Evaluation and Mitigation Strategy (REMS).

The US Food and Drug Administration issued its first approval for a biological product produced by genetically engineered animals.

Congressman John D. Dingell (D-MI) introduced HR 759, known as the Food and Drug Globalization Act of 2009, which would amend the Food, Drug, and Cosmetic Act to address food, drug, and device safety, including registration of producers of drugs and applicable fees, documentation for admissibility of drug imports, country of origin labeling, and the inspection of producers of drugs and active pharmaceutical ingredients (API).

Also, PPD to acquire AbC.R.O.; Bilcare Global Clinical Supplies named Tony Moult general manager of Bilcare GCS Europe; more...

Also, recalls for two KV Pharmaceutical subsidiaries; Human Genome Sciences delivers anthrax drug to US Strategic National Stockpile; Akorn president and CEO leaves the company; more...

The Federal Trade Commission has filed a complaint in federal district court challenging agreements in which Solvay Pharmaceuticals (Marietta, GA) paid generic drug makers Watson Pharmaceuticals (Corona, CA) and Par Pharmaceutical Companies (Woodcliff Lake, NJ) to delay generic competition to Solvay's branded testosterone-replacement drug "AndroGel," a prescription pharmaceutical with annual sales of more than $400 million, according to an FTC press release.