
A recent reader poll focused on predictions for the industry's future.

A recent reader poll focused on predictions for the industry's future.

FDA's role should not be overlooked as it has been in years past.
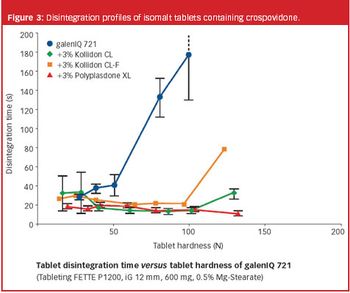
During the past years, there has been increasing demand for fast dissolving disintegrating tablets (FDDTs), such as orally disintegrating tablets (ODTs) and sublinguals.

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Pfizer will purchase Wyeth (Madison, NJ) under a definitive merger agreement approved by both companies' boards of directors.

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...

Achieving double-digit growth through 2011, biotech-based active pharmaceutical ingredients (APIs) are expected to far surpass growth rates for chemically synthesized APIs.

Catalysis for olefin metathesis and aldol reactions and synthetic routes to natural products are some recent gains.

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region's short- and long-term role? This article contains bonus online-exclusive material.

Instead of hampering progress, cost pressures might inspire Big Pharma to get creative.
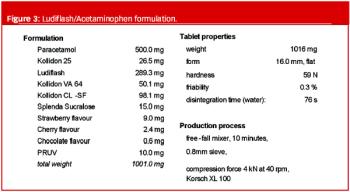
A new excipient for orally disintegrating tablets not only imparts superior tablet characteristics, but has the added advantage of allowing users to maintain full control over their formulations, manufacturing processes and intellectual property.

Also, Crucell and DSM announce deals with GSK, Talecris, and CSL; Nobel Prize winner Luc Montagnier joins Viral Genetics; more...

The US Food and Drug Administration issued a draft guidance, Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches.

Also, BASF opens lab in Mumbai; Evotek president and CEO to resign; more...

At its annual business briefing held last week, Merck & Co. outlined its short- and long-term strategy for growth. Its strategy is focused on increased penetration in emerging markets, the establishment of a business for developing follow-on biologics or biosimilars, and a new commercial model for product life-cycle management.

Also, NicOx and DSM make manufacture and supply pact for naproxcinod drug substance; NovaRx appointed Norrie Russell president and COO; more...

A Centers for Disease Control and Prevention (CDC) study confirmed that severe adverse reactions reported in the fall and winter of 2007 resulted from heparin contaminated with oversulfated chondroitin sulfate (OSCS).

The Synthetic Chemical Organic Manufacturers Association (SOCMA) has raised concerns over requirements for security vulnerability assessments (SVA) under the US Department of Homeland Security's (DHS) Chemical Facility Anti-Terrorism Act Standards (CFATS).

Reps. John D. Dingell (D-MI), current chairman of the Committee on Energy and Commerce in the US House and Representatives, and Bart Stupak (D-MI), chairman of that committee?s Oversight and Investigations Subcommittee, said that moving the Food and Drug Administration Globalization Act and other measures for drug and food safety will be a key priority for the next Congress.

Also, AstraZeneca announces changes to its supply chain operations; Christian Velmer appointed head of Wyeth Canada; More...

A recent study shows life-science companies have difficulty in planning and executing change-management practices.

2009 will likely be a difficult period for emerging biopharmaceutical companies.