
Schott and W.L. Gore introduced a prefillable, silicone-free glass syringe system, which won an award at CPhI Worldwide.

Schott and W.L. Gore introduced a prefillable, silicone-free glass syringe system, which won an award at CPhI Worldwide.

West introduced NovaPure 3-mL Cartridge components and the SmartDose Gen. II 10-mL injector.

The company showcased its compact robotic nest filling machine at CPhI Worldwide 2019 on Nov.–7 in Frankfurt, Germany.

Trends affecting primary pharmaceutical packaging include the shift to more complex drug-device combination products.

The company introduced its new advanced elastomer, the 4040/40 formulation, and a new line extension of its AccelTRA component program, AccelTRA Select, at CPhI Worldwide from Nov. 5–7 in Frankfurt, Germany.

Through a partnership with the Sobti family, the CDMO will expand is injectable dosage form capabilities.

The plant in Singen, Germany will be used for formulation, filling, and packaging of Takeda’s vaccine candidate.

The companies plan to promote and manufacture Maverick, an emergency-use, cartridge-based auto-injector.

The company will showcase its RayDyLyo all-plastic capping solution, which works in conjunction with SCHOTT’s adaptiQ ready-to use (RTU) vials and groninger Nestfillers by providing maximum flexibility for fill/finish operations.

US-based SP Industries acquired the sterile filling line assets of Spain-based i-Dositecno.

Gerresheimer enlarged a cleanroom, installed a high-performance furnace, and automated testing and packaging systems for pharmaceutical glass packaging in Essen, Germany.

The new device can inject a range of drugs of different viscosities up to 35cP and different fill volumes up to 1mL.

Ready-to-use components help compounders and manufacturers of small batches overcome economy-of-scale limitations in aseptic GMP manufacturing.

Optima Packaging explains its approach to process design.

Automated fill/finish and inspection equipment displayed at the 2019 Healthcare Packaging Expo improves quality and efficiency.

Advances in fill/finish for parenteral packaging address demands for efficiency and product safety.

The company will supply its heparin sodium injection in prefilled syringe form.

The Industry 4. 0 tools of augmented and virtual reality aid pharmaceutical fill/finish equipment design, training, and troubleshooting.

Nephron Pharma and the University of South Carolina plan to build a sterile compounding lab at the University for research and training.

Equipment and systems for aseptic transfer and manufacture meet cGMP and Annex 1 requirements for pharmaceutical fill and finish.

The companies will work together to produce enFuse, a patient-administered subcutaneous delivery device.

Optima Pharma opened a new assembly hall, the CSPE Center, that will give the company space to build complete, multi-story pharmaceutical manufacturing facilities.

Nephron Pharmaceuticals is partnering with Clemson University to create a robotic solution for syringe-filling automation to enhance sterile manufacturing.

Glass vials have come a long way from mere commodities and are now considered an integral part of final drug product.
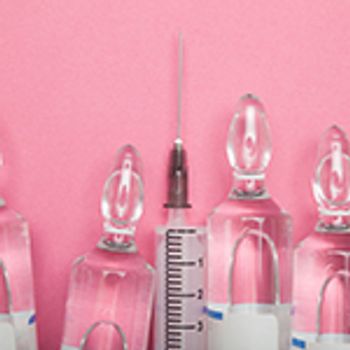
Formulators of parenteral drugs must be cautious of specific considerations and challenges that arise during development and manufacture.