
The expansion will enable relocation of its North American headquarters from Grayslake, Illinois.


The expansion will enable relocation of its North American headquarters from Grayslake, Illinois.

Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

Bill Hartzel, Director Strategic Execution, Advanced Delivery Technologies at Catalent Pharma Solutions, spoke with Pharmaceutical Technology about blow-fill-seal for aseptic processes.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

Vetter completes on-site expansion activities of visual inspection and in-process control at Chicago facility.

With drug development trends shifting towards personalized medicines, new technologies are needed for the manufacture of these highly sensitive drug products.

Lyophilization Services of New England announces inspection approvals that will allow its Bedford, NH facility to begin manufacturing commercial drugs for US distribution.
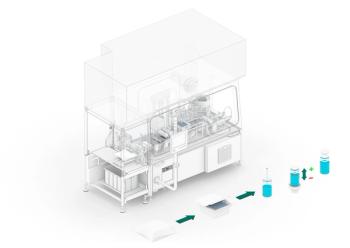
Groninger has developed FlexPro 50, an isolator system for aseptic processing of nested syringes, cartridges, and vials. FlexPro 50 is capable of achieving outputs of up to 5000 containers per hour.

Optima Pharma is exhibiting a range of technologies from sterile-filling and freeze-drying solutions to isolators and restricted access barrier systems.

Machine manufacturer IMA is showcasing a number of technological advances for the processing and packaging of pharmaceutical products at ACHEMA 2015.
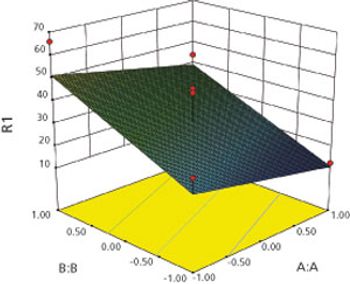
Box-Behnken modeling was used to optimize a resinate complex, to mask the taste of levocetirizine dihydrochloride and montelukast sodium in orally disintegrating tablets.

Symbiosis Pharmaceutical Services is now offering a new bulk lyophilization (freeze drying) service in response to increasing demand in the manufacture of bulk intermediates and APIs by lyophilization.

This article looks at data gathered from several studies of a widely marketed chlorobutyl rubber formulation used for vial stoppers and prefilled syringes.

This study evaluates the impact of controlled nucleation on the ability to optimize a lyophilization cycle for a monoclonal antibody formulation.

The use of disposables requires careful consideration and planning.

The VERISEQ nucleation technology offers a commercially viable technique for cryogenically generating a uniform dispersion of microscopic ice crystals (or ice-fog).

CDMO Vetter produces identifiable labeling for a top-ten pharmaceutical company.
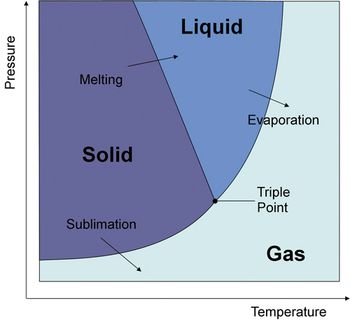
Efficient freeze-drying processes result in time and energy savings, reduced failure rates, and improved batch consistency.

McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.
While the optimization of a lyophilization cycle for a biologic relies on a well-characterized formulation, viscosity and aggregation after product reconstitution must also be carefully managed.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.


Pre-sterilized, nested syringes and vials are seeing increased use in sterile filling.

The acquisition of Aptuit's Glasgow, UK and Indiana, US facilities will add sterile injectable formulation development and expand AMRI's analytical services capabilities.

GSK Australia's Boronia site will install next-generation blow-fill-seal machinery for aseptic filling.