
Primary packaging and manufacturing technologies minimize product/package interaction, protect quality, support safe travel through the supply chain, and enhance performance at point of use.

Primary packaging and manufacturing technologies minimize product/package interaction, protect quality, support safe travel through the supply chain, and enhance performance at point of use.
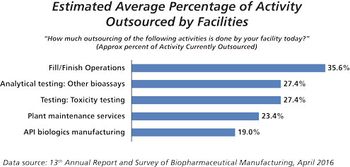
This key bioprocessing segment is expecting continued growth

Visual inspection of parenteral vials is the first step in a root cause investigation.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

Dalton Pharma Services completed an expansion in sterile filling and API manufacturing at its cGMP facility in Toronto, Canada.

Recipharm is investing EUR3.7 million (approximately $4.18 million) to increase lyophilization capacity at its Masate facility in Italy.
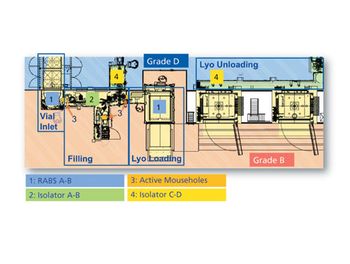
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

A technology management process identifies and evaluates new technologies in biopharmaceutical manufacturing to aid business decisions.

The company manufactures biological drug products and intermediates for the allergy vaccine market.
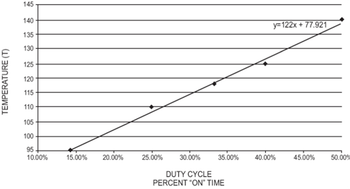
Preheating pinch valves prevents drift in the volume of liquid dispensed.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.

Intact enables aseptic filling in non-classified environments, maintains sterility throughout the process, and reduces costs, timelines, and contamination risks.

Fogg Filler’s enclosures for bottle-rinsing machines have improved fume containment and doors with lift-off hinges for maintenance.

Troubleshooting and collaboration are essential in implementing commercial lyophilization processes.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.
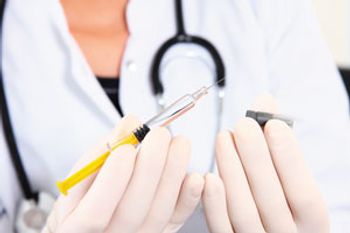
Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

The revised USP Chapter 1207 gives best practices for obtaining reliable data in container closure integrity testing.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.

Although shortages, quality, and regulatory challenges remain, improved technologies and new investments suggest that the worst may be over.

Miriam Beyer, European marketing manager, West Pharmaceutical Services, describes causes of recent parenteral drug shortages.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

Ajinomoto Althea, a biopharmaceutical CDMO, is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates (ADCs), the company announced on Oct. 14, 2015. The new facility is located in close proximity to the company’s existing operations in San Diego, CA.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.