Manufacturing
Latest News
Latest Videos

More News

The launch of Roche's cobas mass spectrometry solution will bring fully automated mass spec analysis to the clinical lab.

Cytiva's Nicolas Pivet, vice-president and general manager, Technology Solutions, emphasizes how automation benefits process development, particularly in the analytical space.

The companies will use Orexo’s powder-based drug delivery technology to develop mucosal vaccines in an inhaled formulation.

Scinai Bioservices Inc. has been established in Delaware as the company's new US-based subsidiary, which will serve biotech companies in early stage drug development.

Novo Nordisk will use the DKK 8.5 billion (US$1.2 billion) to build a new modular and flexible production facility in Odense, Denmark, that will produce multiple products for rare diseases.

Pharmaceutical Technology sits down with Christian Dunne from ChargePoint Technology to discuss the key processing equipment trends in the bio/pharma industry.

In the second part of a video interview, Colin McKinlay, PhD, senior director, Chemistry and Delivery Technologies, at Nutcracker Therapeutics, discusses trends and the future direction of mRNA–LNP development.

Colin McKinlay, the senior director of Chemistry and Delivery Technologies at Nutcracker Therapeutics, discusses current challenges in mRNA and LNP manufacturing as well as innovations that meet these challenges.

With this investment, BioMarin will add a new laboratory to its Shanbally, Co. Cork, Ireland, manufacturing facility.

The European Commission has approved Novo Holdings' acquisition of Catalent, which includes the related sale of three manufacturing sites to Novo Nordisk, which is also acquiring the Czech Republic manufacturing site of Novavax for $200 million.

This paper explores the legal and regulatory framework around 3D drug printing, particularly for personalized medicine, considering regulatory compliance, business concerns, and intellectual property rights.
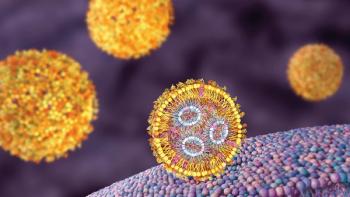
LNPs have gained solid ground as a drug delivery system for mRNA due to their success in the vaccines arena.

Lilly is investing $3 billion to expand its recently acquired manufacturing facility in Wisconsin, while Amgen is investing $1 billion to expand its facility in North Carolina.

Through the agreement, Cambrex will provide accelerated access to clinical development capabilities to Lilly’s biotech collaborators.

EXO Biologics and ExoXpert, an EXO Biologics subsidiary, have received GMP certification of a European manufacturing facility for exosomes and have successfully loaded mRNA and DNA payloads into GMP-grade exosomes for drug delivery.
Edwin Stone, Bernard Sagaert, and Khaled Yamout go behind the headlines to discuss what the ongoing measles outbreak in the UK means for pandemic preparedness and anti-vaccination trends; new mRNA approaches; and what Roche’s acquisition of Poseida means for complex cell therapies.

The ROSS Model 42N-10 has a working capacity of 10 cubic feet and is designed for free-flowing materials with bulk densities up to 100 lbs per cubic foot.
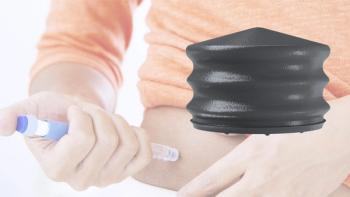
Datwyler has launched new coated plungers in its NeoFlex line that are suitable for large volume biologics.

Through this acquisition, the German CDMO expands its global network and adds aseptic fill/finish and lyophilization expertise to its sterile manufacturing capabilities.

FDA's approval will allow Kedrion to manufacture Ryplazim (plasminogen, human-tvmh), the only FDA-approved therapy for treating PLGD-1, at facility in Bolognana, Italy.

The Thousand Oaks, Calif., cell therapy manufacturing facility now houses new production suites, updated development labs, and more after expansion.

The $3.6 million investment will allow the CDMO to boost its advanced labeling, automated visual inspection, and fill/finish capabilities.

Sanofi will use this investment to increase its antibody bioproduction at its site in Lyon Gerland, France.

Webinar Date/Time: Thu, Dec 5, 2024 11:00 AM EST

The companies will collaborate to create and test circVec DNA–LNP formulations for use in potential therapeutic applications.