
Catalent's Advasept platform uses blow-fill-seal technology to aseptically manufacture, fill, and seal a polymeric primary container for injectable drugs.

Catalent's Advasept platform uses blow-fill-seal technology to aseptically manufacture, fill, and seal a polymeric primary container for injectable drugs.

MG America's ACE-BT300 Coding and Verification Unit provides track-and-trace capabilities for bottles.

The FXS Combi from Bosch Packaging Technology features an integrated capping station for vials and cartridges.

The Press Out Universal Mini from Sepha can deblister at a speed of up to 15 packs per minute.

The ionHP biodecontamination hydrogen peroxide-based sterilization technology is designed for use in aseptic enclosures.

The Manesty TPR 500 tablet press from Bosch Packaging Technology increases output and features a hygienic design.

New identifiers and tracking requirements aim to block illegitimate products.
Common challenges and key considerations when developing a freeze-drying cycle for protein pharmaceuticals.
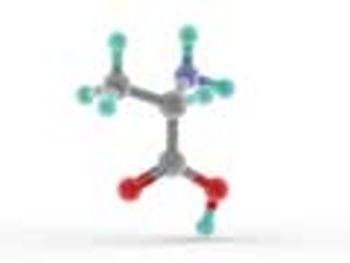
USP's Therapeutic Peptides Expert Panel discusses manufacturing processes and impurity control for synthetic peptide APIs.

Pharmaceutical Technology spoke with Gloria Gadea-Lopez, associate director of Shire, and Martin Dittmer, PharmaSuite product manager at Rockwell Automation, about how manufacturing execution systems (MES) can improve productivity and the current challenges faced by companies implementing MES systems.

New formulations and expanded vaccine production are encouraged.

Gallus BioPharmaceuticals enters a cell line optimization and manufacturing agreement with Omni Bio Pharmaceutical.

TAP Biosystems announced that Gallus BioPharmaceuticals is using its ambr15 micro bioreactor system to optimize process development and clone selection of novel antibody therapeutics and biosimilars.

CPhI report warns industry must actively drive process improvements to meet growing global demand for pharmaceuticals.

The cleaning validation lifecycle includes assessment, development, validation, and monitoring.

Both upstream and downstream processes can benefit from continuous manufacturing advantages.

Tony Wright, CEO of Exelsius Cold Chain Management, answers questions about the new requirements for temperature-controlled pharmaceutical distribution.

Doug Hausner, Engineering Research Center for Structured Organic Particulate Systems, Rutgers University discusses continuous manufacturing for solid-dosage drug production.

The author discusses various aspects of the hot-melt extrusion process and outlines a practical approach to scale-up.

The authors describe how modular systems can be applied to continuous solid-dosage and nutraceutical manufacturing.

Charlie Martin, Leistritz Extrusion, describes hot-melt extrusion manufacturing processes and advances.

The author examines the effect of compaction force, gap width, and sieve setup on granule size.

The selection of excipients is important in generic formulations due to the impact it has on the risk and performance of generic drugs.

The AdvantaPass wall pass-through system from AdvantaPure permits aseptic transfer of fluids between suites.

A universal Milling Isolator from Powder Systems Limited (PSL) handles multiple mills.