
A changing biopharmaceutical industry is going beyond typically outsourced activities and is using CMOs for more challenging processes. Review the top 10 outsourcing trends.

A changing biopharmaceutical industry is going beyond typically outsourced activities and is using CMOs for more challenging processes. Review the top 10 outsourcing trends.

PharmaCell enters an agreement purchase TiGenix therapy production facility.

I Holland announces service that predicts solutions for tablet sticking problems.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

Caron's New Environmental Test Chambers and Refrigerated Incubators

Products enable testing in accordance with methods described in new USP monograph.

A packaging defect has led to Merck voluntarily recalling all lots of Liptruzet tablets in the United States.

A low-profile pneumatic pharmaceutical conveyor moves tablets and capsules in restricted height areas.

A survey of BioPharm International readers found that single-use systems and other technologies are driving process efficiencies in biomanufacturing, but there is room for improvement.

Consider these best practices when deciding to implement single-use components.

PCI discusses the challenges and benefits of serialization in light of the new US track-and-trace legislation.

MedImmune has entered into a research collaboration with Immunocore to develop novel cancer therapies.

CS Inspection Machine Improves Efficiency

Ribbon Blender Model 42N-270 Increases Performance

ALpHA G Capsule Filter for Single-Use Systems

Training, calibration, and preventive maintenance help prevent over-and under-weight tablets. The author discusses causes of off-weight tablets and best practices for tableting.

Gericke N200 Nibbler Provides Size Reduction

Different approaches for qualification of personnel for visual inspection of residues on equipment surfaces are reviewed.
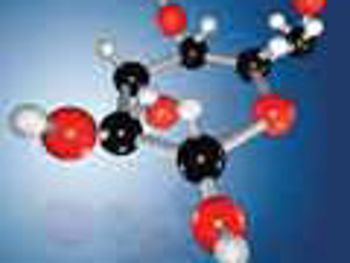
NMR analysis provides crucial structural information of synthesized glycans while LC-MS/MS is ideal for quantitation of free sugars in biological matrices.
Antibody fragments pose unique challenges in terms of recovery, purification, and formulation.

Kurt Lumden, Director, Client services at PAREXEL's Perceptive Informatics, discusses the management of investigational product temperature excursions.

With SMEs gaining wider recognition as the powerhouse of research and innovation in Europe, regulatory agencies are urging companies to engage with regulators early in the drug-development process.

CMC Biologics and OnoSynergy form an agreement from cell-line development.

Pfizer appoints management changes to take effect in 2014.

Cyclonic-spray technology improves spray consistency and allows mixing at the point of delivery.