
The Drug Quality and Security Act creates national standards for serialization of drug products to protect against counterfeiting.

The Drug Quality and Security Act creates national standards for serialization of drug products to protect against counterfeiting.

Covance Inc. and Pathoquest have announced a collaboration to provide next-generation sequencing (NGS) based biosafety assessments to detect and identify viral contaminants within biologic compounds.

Ei Lilly announces investments in insulin manufacturing capacity for its sites in Indianapolis, Puerto Rico, France, and China.

Intertek is now offering comprehensive services for the development of orally inhaled and intranasal drug products (OINDPs) for both small molecules and biologics.
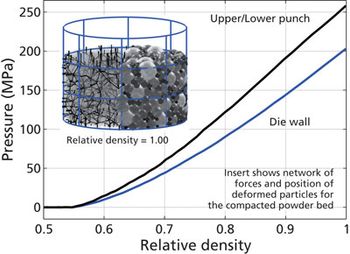
Screening methods and predictive models address tenacious tablet-sticking problems.

The systems are designed for the manufacture of oncology products and to research new biotech products.

Changes in the working procedures and disposable fluid flow paths resulted in a measurable decrease in product waste in a low-volume, high-value fill-finish line.

Schott’s glass vials are specially manufactured to control and reduce delamination potential.

Progress in delivery science, manufacturing technologies, and commercialization are playing critical roles in advancing the development of complex parenteral drug formulations for new drug substances having a variety of formulation challenges

AstraZeneca will build a new facility in the United Kingdom to produce Zoladex, an injectable prostrate cancer treatment.

Grifols expands and diversifies portfolio with acquisition of diagnostic business.

Breakthrough Therapies Raise Manufacturing Issues

ISPE's survey is industry's first large-scale effort to collect data on patient experiences with clinical trial materials.

Bend Research adds powder-filling technology.

The European Pharmacopoeia Commission has decided to change its approach on elemental impurities.

Automation System Improves Operational Insight

Data from BioPlan Associates’ 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.

Legislators agree on a limited bill affirming FDA authority over compounders while setting up a process for national drug tracking.

Sterilization validation must be based on a robust, quality-by-design philosophy; autoclave overkill cycles should be validated by correlating lethality data to support chosen critical process parameters, even when using overkill sterilization.
Wireless Network Monitors Air Cooled Heat Exchanger

Database Improves Process Efficiency
System Links Packaging Operations

Novartis files Citizen Petition with FDA to maintain naming policy for all biologics to help ensure patient safety.

GSK and the Gates Foundation will invest a combined $1.8 million in early stage research into vaccine thermostability.

The Parenteral Drug Association has established a task force to develop a peer- and regulatory agency-reviewed Technical Report that will serve as a science-based industry reference document.