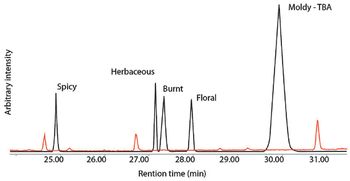
Combining olfactometry analysis with multidimensional gas chromatography–mass spectrometry provides an extremely useful analytical method for identifying aroma or odor notes from a sample.

Combining olfactometry analysis with multidimensional gas chromatography–mass spectrometry provides an extremely useful analytical method for identifying aroma or odor notes from a sample.
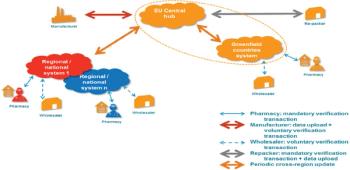
Counterfeit medicines are an increasing threat to the EU supply chain and there is need for a standard identification solution across Europe.

A Technical Forum Moderated by Patricia Van Arnum, featuring contributions from PerkinElmer, BioTools, Chiral Technologies, Shimadzu Scientific Instruments, GE Analytical Instruments, and Waters.
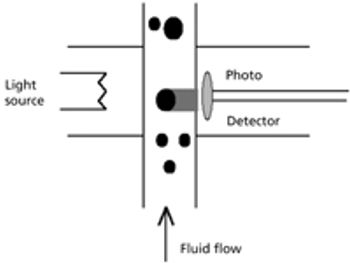
This article focuses on the history of glass delamination and methods that detect it, both from a compendial and a research perspective.

The authors discuss the analysis of the resulting data, focusing on methods for the calculation of mass median aerodynamic diameter, one of the metrics routinely used for comparative testing.
The authors investigate the effect of low pH and ionic strength on aggregation using turbidity measurements and size-exclusion–high-performance liquid chromatography.
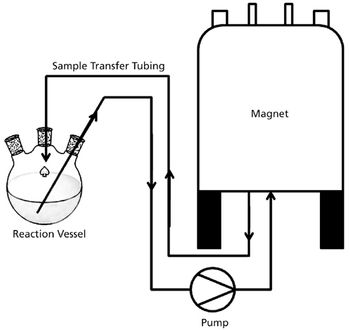
The authors describe the benefits of nuclear magnetic resonance (NMR) compared with traditional monitoring techniques. They also discuss how NMR reaction monitoring provides a new process analytical technology tool for industry.

Continuous manufacturing is increasingly noted as an important long-term objective for the pharmaceutical industry. PTE talks with Tim Freeman, Director of Operations at Freeman Technology, about some of the central issues involved in this transition, as well as the supporting role of relevant analytical technology.

It is very difficult to measure the problem of counterfeiting accurately from year to year; by definition criminals don?t file tax returns or publish quarterly earnings.

In an age dominated by the internet and uncertainty over the best packaging security methods to employ, counterfeit medicines have the ideal environment to thrive.

Inspection systems play a big part in ensuring product quality.

PTE interviews Leigh Jordan, UK Sales Manager at Cognex UK Ltd, about the importance of code readability when using anticounterfeiting solutions such as 2D datamatrix codes.

When I run a small production batch of a particular formulation with the same tablet press used to develop it, I get compressibility and disintegration issues. What am I doing wrong?

Recent recalls have contributed to the pharmaceutical industry?s heightened awareness of glass delamination (i.e., the formation of glass flakes in a vial), which could affect drug quality and patient safety. To confront this growing problem effectively, drugmakers must understand its causes.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the October 2011 edition from Edwards and Moyno.

The future, and increasingly the present, for aseptic operations is isolated robotics. Companies that wish to gain competitive advantages in their operations are stepping up and taking notice.

EMA released two concept papers for consultation that address the need to revise existing guidelines on biosimilar medicines and influenza vaccines.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

Europe establishes new collaborative system to track products.

AAPS Global Health Focus Group's Kishor M. Wasan discusses new initiatives.
Single-use components in the fill–finish line provides increased flexibility to multiproduct manufacturers.
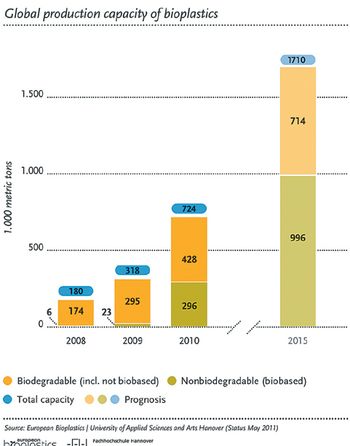
Drugmakers and packagers are pursuing various initiatives to reduce their carbon footprints. This article contains bonus material.

Getting the most value out of M&As requires proper upfront legwork.

Corporate management must be held accountable for quality at all levels.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.