
Contract manufacturing organizations throughout Asia are increasing their capabilities to meet market demand and attract foreign investment and partnerships.

Contract manufacturing organizations throughout Asia are increasing their capabilities to meet market demand and attract foreign investment and partnerships.

Are biosimilars the next big thing or just the next big bubble?

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.
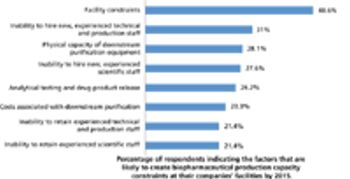
China rises to the top as a destination for international outsourcing.

A book about developing quality-control training manuals provides a wealth of information and a dearth of practical help.

New product reviews for August 2011 focusing on automation, process control, and information technology.
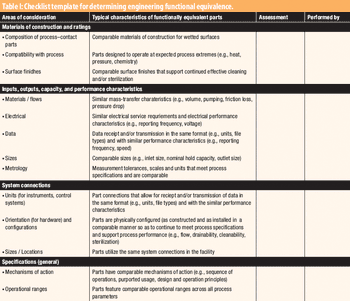
The second in a series of eight case studies from the Product Quality Research Institute focuses on functional equivalence for equipment.

Visiting a new site or going down memory lane may not get you where you want to go.

New studies reveal the promise and feasibility of transdermal vaccine delivery.

Many child-safe package designs help improve compliance and provide tamper evidence.

This study demonstrates the beneficial use of a spatial-filter velocimetry particle-size analyzer during granulation.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.
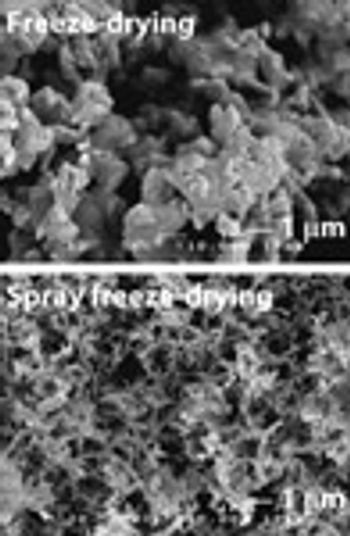
The authors discuss the preparation of lipophilic drug nanocrystals by controlled crystallization during freeze-drying.

Quality management requires more effort in a complex supply chain.

Thomas P. Layloff describes the advantages of using thin-layer chromatography methods for counterfeit detection. This article contains bonus online material.

Analytical detection techniques help combat counterfeit drugs.

A path to personalized medicines creates a new paradigm for development and manufacturing.

USP promotes horizontal standards for biologics' quality attributes.

While most companies recognise the significance of emerging markets, they struggle to jump the first hurdle in addressing these opportunities: defining the right product.

Manufacturers are facing ever-increasing competition while end users are expecting better value and efficacy. The authors discuss these challenges and offer practical solutions.

The authors review current industry challenges and trends in managing global supply chains and propose best practices for improving visibility into those networks.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.
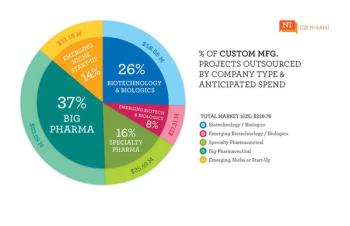
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

The authors share their approach and experience working in complex, multicompany environments for in-licensed products to develop successful chemistry, manufacturing, and controls packages for managing outsourcing partnerships.

Graham Rideal of Whitehouse Scientific explains the importance of filter testing and offers some considerations with regards to choosing filter test methods.