
In an effort to balance bilateral trade, India is urging China to increase Indian pharmaceutical imports.

In an effort to balance bilateral trade, India is urging China to increase Indian pharmaceutical imports.

New product reviews for October 2011 focusing on analytical instrumentation.

Manufacturers fund research and reduce prices to tackle diseases.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Despite new hurdles, industry must move forward and fulfill its mission.

This risk-management case study focuses on assessing empty capsules.

In a quick-fire interview, Dr Stephen Taylor, Vice President & Commercial Director at Fujifilm Diosynth Biotechnologies, looks at some of the challenges facing biologics outsourcing.

GlaxoSmithKline (GSK) is looking to racecar technology as it seeks to improve its manufacturing, R&D, and consumer-healthcare areas. The company has formed a long-term partnership with the UK-based McLaren Group, which is best known for its expertise in the Formula 1 motor sport.

GE Healthcare, the health business of General Electric, will dedicate $1 billion of its total R&D budget during the next five years to its technologies for manufacturing biopharmaceuticals and for cancer research. Part of the money will go toward expanding the company's cancer-diagnostic and molecular-imaging capabilities, as well.

President Obama released his plan for deficit reduction on Sept. 19, 2011, and included in the 80-page report are several provisions that would affect US drug makers if enacted.

In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Lechler and National Bulk Equipment.
Glass flaking or delamination can result in a failed quality-assurance test, thus bringing production to a halt and causing substantial revenue loss. If glass delamination remains undiscovered, it can pose a serious contamination risk to the drug product and a potential health risk to the public.

In our manufacturing process, we are running into issues with our vial stoppers clumping in the feeder bowl. How can we ensure that the stoppers go through smoothly without clumping up?

The International Society for Pharmaceutical Engineering will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide and contain practical information about technological advances in sterile manufacturing.

Powder Systems Ltd's (PSL's) new GFD™ isolator combines the latest innovation to provide a high containment lab scale filtration and drying solution with the same reliability and benefits as PSL high containment production size filter dryers.
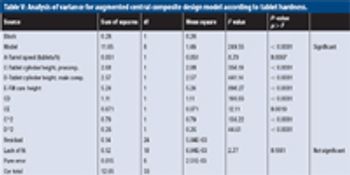
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.

A Q&A with John Plachetka, chair, president, and CEO of POZEN, on recent industry trends.
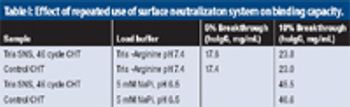
A new, robust method for protein elution from ceramic hydroxyapatite.
PDUFA renewal legislation sets stage for new policies affecting revenue, resarch, and oversight.

Being aware of a forthcoming inspection or how a product was made can make a huge difference.

A report commissioned by FDA evaluates the QbD program.
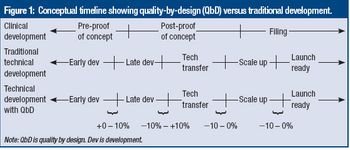
Directors from FDA's Center for Drug Evaluation and Research summarize findings in an FDA-commissioned report on QbD and propose actions the agency can take to encourage full-scale QbD implementation.

Growth and change in Brazil and Mexico offer key opportunities for the region's pharmaceutical industry.

The author offers perspectives on ways in which pharmaceutical companies and other stakeholders in the supply chain can confront the threat of counterfeit products, cargo theft, illegal diversion, and economically motivated adulteration.