
Which route will we take to arrive at a national stem-cell policy?

Which route will we take to arrive at a national stem-cell policy?
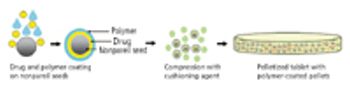
The current review describes the role and selection of excipients, pellet core, coating materials, and compression with various cushioning agents.

Switzerland is an important power in Europe's pharmaceutical industry.

Product degradation is often overlooked compared with other supply chain issues, but if left unchecked can lead to the market entry of substandard medicines, whether through ignorance or gross negligence.

Lyophilisation or freeze-drying is widely used in the pharmaceutical industry for a variety of reasons.

Single-use filtration and fill–finish technologies can be used as part of a lean manufacturing strategy to boost production, and reduce manufacturing waste and costs.

President Obama unveiled an Advanced Manufacturing Partnership designed to reinvigorate the country's manufacturing sector.

The IPEC Federation has issued a statement on the use of phthalates in pharmaceutical products in response to reports of adulteration of certain nutritional supplements, vitamins, foods, and beverages imported from Taiwan.

The traditional method of conveying information in the brief summary of a printed prescription-drug advertisement is neither the most comprehensible nor the most preferred by consumers, according to an FDA study.

FDA released a new strategy on that is aimed at meeting the challenges posed by rapidly rising imports of FDA-regulated products and the growing complexity of the pharmaceutical supply chain.

Novartis has commenced construction of a new manufacturing plant in Russia that represents the company's most significant investment in the country to date.

GlaxoSmithKline has entered into an agreement to purchase Shenzhen Neptunus? stake in a previously formed joint venture between the companies involved in the development and manufacture of influenza vaccines in China, Hong Kong, and Macau.

Merck and Hanwha Chemical have formed an exclusive global agreement to develop and a commercialize a biosimilar of Enbrel, a drug to treat moderate to severe plaque psoriasis, psoriatic arthritis, and moderate to severe rheumatoid arthritis.

After looking back at the first year of its Bad Ad outreach program, FDA judged that the initiative has successfully raised awareness about misleading promotion, according to an FDA press release.
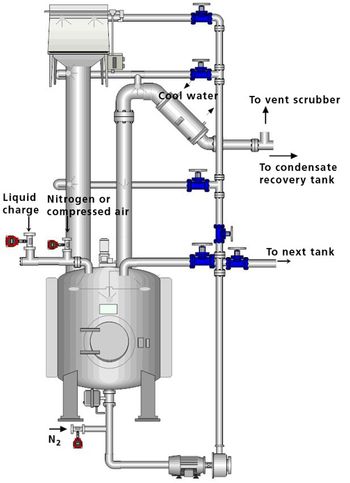
The author describes the benefits and challenges inherent to cleaning in place (CIP). The article also describes the development and validation of a CIP cycle.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2011 edition from Meissner and Telstar.

Our company is getting low and unpredictable cycle life out of our diaphragm valves. This problem has caused us to implement standard operating procedures for frequent replacement of the diaphragms, which is costly and time consuming. What could be happening, and what are our options?

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

AstraZeneca agreed to settle a sex-discrimination lawsuit by paying $250,000 to 124 women who worked at the company's Philadelphia Business Center.

Can microdosing make medicines safer and more effective for children?
More sophisticated biological expression systems expand the functionality of the traditional systems for protein synthesis.
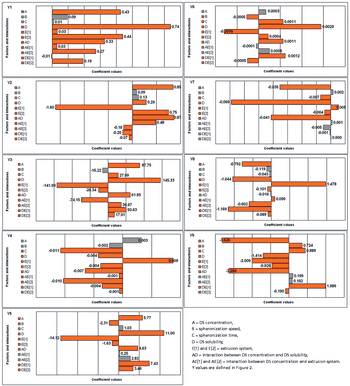
The authors compare three systems of single-screw extrusion using binary formulations for their suitability for producing pellets of various formulations and under various spheronization conditions.

Academic–industry partnerships are increasingly important in biopharmaceutical innovation.

Editor's picks of new manufacturing products for June 2011.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.