
McNeil Consumer Healthcare, a division of Johnson & Johnson (J&J), has once again expanded the recall of certain OTC products because of a musty or moldy odor, which has been linked to trace amounts of the chemical 2,4,6 triburomoanisole (TBA).

McNeil Consumer Healthcare, a division of Johnson & Johnson (J&J), has once again expanded the recall of certain OTC products because of a musty or moldy odor, which has been linked to trace amounts of the chemical 2,4,6 triburomoanisole (TBA).

Merck & Co. (Whitehouse Station, NJ) released details of a restructuring plan last week, which calls for phasing out operations at eight research sites and eight manufacturing sites, resulting in a 15% reduction of its global workforce.

The Council of Europe (CoE) is hoping its Medicrime treaty can help curb the lucrative global trade in fake medicines.

The Pharmaceutical Research and Manufacturers of America (PhRMA) announced earlier this week that John J. Castellani will replace Billy Tauzin as President and Chief Executive Officer.

Last week, Senator Charles Grassley (R-IA) sent letters to 16 drugmakers, including Pfizer (New York), AstraZeneca (London), and Eli Lilly (Indianapolis), asking them about their current policies regarding whistleblowers?employees who file complaints under the False Claims Act (FCA).

Eli Lilly to Acquire Alnara Pharmaceuticals; Exelixis CEO Leaves for Biogen Idec; And More.

The US government outlines its strategy against counterfeiting and intellectual-property infringement, including ways to better secure the pharmaceutical supply chain.

The authors argue that the cost of generating nitrogen via an in-house gas generator is considerably lower than the cost of using fractional distillation to generate liquid nitrogen.

Duke University researchers have found a possible alternative to lyophilization.
Nanotechnology is an important area of drug and biomedical research, and advancing nano-analysis is crucial for its further development.

To continue innovating, the biopharmaceutical sector needs support from all levels.

As generic divisions become the most-wanted acquisitions of Big Pharma, India's domestic industry may be thinning out.
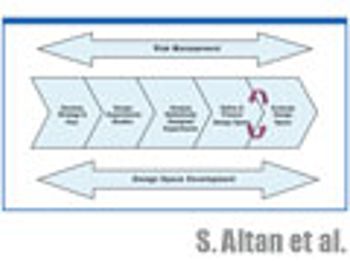
The authors discuss the statistical tools used in experimental planning and strategy and how to evaluate the resulting design space and its graphical representation.

Editors' picks of pharmaceutical science and technology innovations.

Rising labor costs in China may result in increased competition for higher value services.

Is now the time for multiparticulates to shine as a controlled-release solution?

Technical Note: The authors investigated the variables important for calcium-alginate formation as well as dissolution.

Reports of overlooked controls, dropped pallets, and misplaced documents leave a chill in the air.

After a spate of industrial disasters, the public seeks greater oversight of corporations-so does FDA.

More information may be released to improve public understanding of regulatory policies.

Pfizer Suspends Tanezumab Program; Actavis Appoints CEO; And More.

The effects of counterfeiting are hard to measure, both in human impact and financial loss.

Xavier Parissaux of Roquette explains why the pharma industry's need to reduce organic solvent use is driving innovation in film coatings.

X-ray powder diffraction (XRPD) is a versatile, non-destructive technique that reveals detailed information about pharmaceuticals.

With the increasing financial and technical means of counterfeiters, the number of counterfeit pharmaceuticals in the supply chain is growing at an alarming rate.