
sanofi-aventis outlined an investment plan for adapting its chemical and biotechnology manufacturing facilities in France during the next four years.

sanofi-aventis outlined an investment plan for adapting its chemical and biotechnology manufacturing facilities in France during the next four years.

The US Pharmacopeial Convention welcomed Minghao Zhou of China's Zhejiang Provincial Institute for Food and Drug Control.

Eli Lily Wins Court Battle; Genzyme Appoints COO; And More.

CMOs' business model has all of the flaws of the captive model it is meant to replace.

Collaboration has been key to the pharmacopeia's achievement.

Tablets and capsules are mainstay product forms, so what are the spending and innovation trends for solid-dosage manufacturing equipment and machinery?

As drug manufacturing and standards grow, pharmacopeias must adapt to meet new challenges.

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.

As the industry continues to evolve, do we know where we're headed?

An established show has several new programs that reflect the industry's current challenges.

Laboratory personnel share interesting tales as well as stories of unexpected tails.
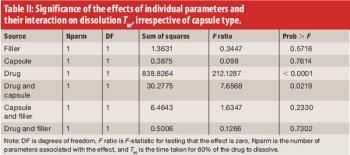
The authors examined the disintegration and dissolution profiles of propranolol and rofecoxib tablets overencapsulated with standard hard-gelatin capsules and with capsules specifically designed for double-blind clinical trials.
Editors' Picks of Pharmaceutical Science and Technology Innovations

As demand for global vaccine development and production grows, all eyes are turning to Asia.

The authors describe a novel approach for assessing method robustness. This article contains online-bonus material and was copublished with Pharmaceutical Technology Europe.

Interphex Showcase 2010.

The BIO convention, and healthcare reform, could re-energize biotech.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and types of spending made in 2009 and planned for 2010.

The authors describe the origins of single-use components and explain their application to aseptic processes. They also show how disposable devices have changed over time and offer a glimpse of the future.
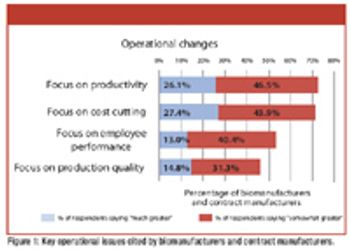
Restructuring in the biopharmaceutical industry is renewing a focus on resource optimization.

FDA lacks resources to manage expanding postmarketing responsibilities.

Steve Sirabian discusses challenges, trends, and more.

Growth in the market for monoclonal antibodies, recombinant proteins, and vaccines creates new opportunities for drug companies and suppliers.

Aseptic blow–fill–seal minimizes human intervention in the packaging process.

New information improves an organization's guide to building manufacturing facilities.