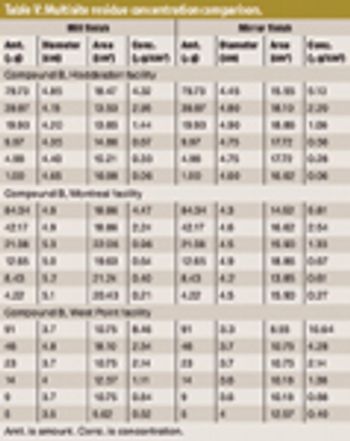
The author tests the ruggedness of VRL viewing conditions and defines optimal viewing conditions.

The author tests the ruggedness of VRL viewing conditions and defines optimal viewing conditions.

An updated book provides essential information for scientists who monitor microbial quality.
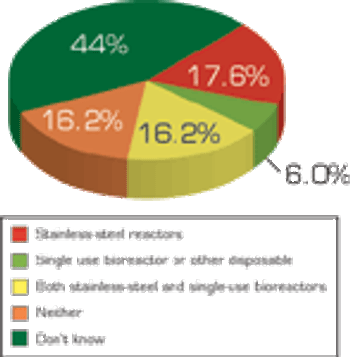
Pharmaceutical Technology's annual survey on equipment and machinery shows fewer companies increased spending in 2008 and still fewer will increase spending in 2009 as overall economic conditions affects purchasing decisions.
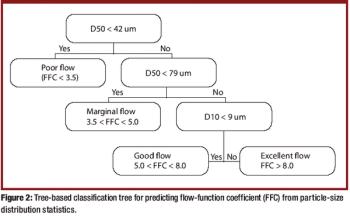
The authors present a simple and material-sparing approach for estimating the powder-flow performance of previously uncharacterized single-component bulk powders when only particle-size distribution data are available.

With the economy down and multinational firms capitalizing on patents in developing countries, India's R&D sector still has a long way to grow.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region?s short? and long?term role?
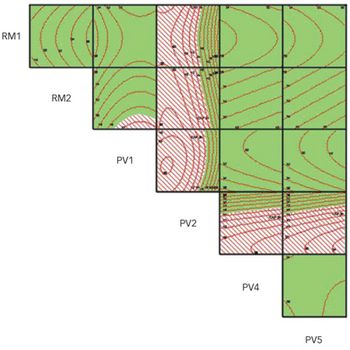
When applied as part of a structured approach, predictive modelling can provide deep process and product understanding, and can enable true, continuous process validation as envisioned by ICH guidelines.
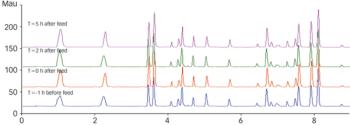
PAT guidance has been available from FDA for more than 4 years, but there have been no apparent breakthroughs in large-scale upstream production. Will companies consider using on?line chromatography to change this?
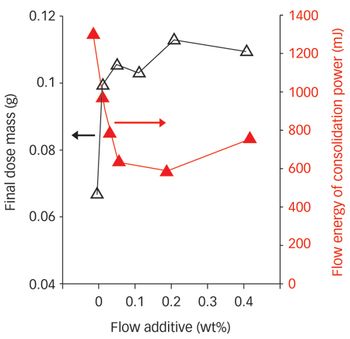
Enshrined in the concept of Quality by Design is the premise that optimized pharmaceutical manufacturing requires detailed understanding of products and processes. With this in mind, many benefits can be achieved by combining modern powder characterization techniques with real processing experience.
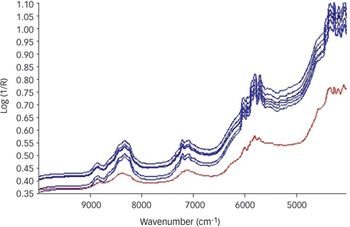
This month's expert examines the most appropriate technique for checking raw material quality. What technique would you recommend for quality checking of raw materials?

Also, GPC Biotech AG and Agennix to merge; BASi appoints COO of scientific services; more...

The United States Trade Representative (USTR) is seeking documentation from chemical companies to identify possible non-tariff trade barriers, created by the European Union's Registration, Evaluation, Authorization and restriction of Chemicals (REACH) regulation, which would be inconsistent with the EU international trade obligations under World Trade Organization (WTO) rules, according to an informational release by the Synthetic Organic Chemical Manufacturers Association (SOCMA).

The US Food and Drug Administration posted the Prescription Drug User Fee Act (PDUFA) IV Drug Safety Five-Year Plan on its website.

After two years of hearings, more than 5000 pages of expert testimonies, and 939 medical articles, a special federal court ruled that there was little, if any, evidence to support the claim that substances in the measles, mumps, and rubella vaccine (including the use of thimerosal) had led to the autism of three children.

Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

Sanofi Aventis CEO Chris Viehbacher provided the company's growth strategy in light of changing conditions facing the pharmaceutical industry, which include patent expirations and declining research and development (R&D) productivity.

Innovative containment solutions aim to minimize workers' exposure to cytotoxic compounds and ensure these compounds are not contaminated.

To implement QbD and reduce business risks, teams should begin QbD collaboration early during process development.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the February 2009 edition from Oystar USA and Schreiner MediPharm.

Taro Pharmaceuticals received a warning letter from the US Food and Drug Administration last week regarding its Brampton, Ontario, manufacturing facility.

The US Food and Drug Administration issued its first approval for a biological product produced by genetically engineered animals.

Congressman John D. Dingell (D-MI) introduced HR 759, known as the Food and Drug Globalization Act of 2009, which would amend the Food, Drug, and Cosmetic Act to address food, drug, and device safety, including registration of producers of drugs and applicable fees, documentation for admissibility of drug imports, country of origin labeling, and the inspection of producers of drugs and active pharmaceutical ingredients (API).

Also, PPD to acquire AbC.R.O.; Bilcare Global Clinical Supplies named Tony Moult general manager of Bilcare GCS Europe; more...

Senior Senate Judiciary Committee members Herb Kohl (D-WI) and Chuck Grassley (R-IA) reintroduced the Preserve Access to Affordable Generics Act to prohibit patent settlements under which manufacturers of branded pharmaceuticals pay generic-drug companies to delay the introduction of generic products to the market.

The United States Pharmacopeial (USP) Convention is pursuing greater collaboration with the US Department of Health and Human Services (HHS), and specifically, the US Food and Drug Administration.