
During the past decade, big pharmaceutical companies have been making major efforts to restructure their manufacturing networks. In their haste to shed costs and assets, however, some may be adding risk to their product supply chains.

During the past decade, big pharmaceutical companies have been making major efforts to restructure their manufacturing networks. In their haste to shed costs and assets, however, some may be adding risk to their product supply chains.

Are manufacturers ready to deal with the economic and logistical challenges that accompany tailored therapeutics?
A number of commentators during the past few years have speculated that nanotechnology is the wave of the future in biotech and pharma.

Tablets, PTK Tablet Presses, Surepharm Services

Successful Cooperation Yields Anti-Stick Rewards

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

Generic-drug and specialty pharmaceutical manufacturer Watson Pharmaceuticals (Corona, CA) agreed to acquire the generic-drug company Arrow Group for $1.75 billion in cash and stock.

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

Following the World Health Organization's declaration last week of an influenza pandemic, vaccine makers in Europe and China as well as world health agencies are stepping up efforts toward rapid development and approval of an effective vaccine.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Bullard and Teledyne Tekmar.

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

Kemwell (Bangalore, Karnataka, India) plans to build a new biopharmaceutical manufacturing plant in Bangalore, India, in a strategic collaboration with Boehringer Ingelheim (BI, Ingelheim, Germany), according to a Kemwell press release.
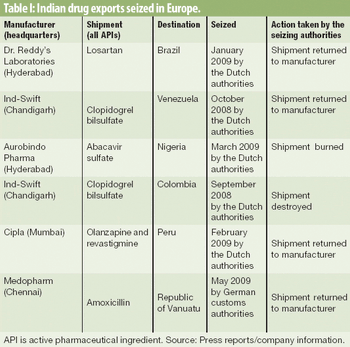
Patent infringement claims and a lack of clear global trade distribution routes may be unraveling the country's generic-drug export industry.

The US Government Accountability Office recommended that the US Food and Drug Administration draft a plan, including milestones, for developing its Sentinel system and ensuring the privacy and security of patients' healthcare data.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

The chief operating officer (COO) of Mylan (Canonsburg, PA), one of the world's leading generic drug manufacturers, has condemned the launch of authorized generic drugs during 180-day exclusivity periods.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

The US biotechnology industry reached aggregate profitability for the first time ever in 2008, representing the only bright spot in an otherwise dismal year for biotechnology financing and performance.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

Members of the House Committee on Energy and Commerce, Chair Emeritus John D. Dingell (D-MI), Chair Henry A. Waxman (D-CA), and Reps. Frank Pallone (D-NJ), Bart Stupak (D-MI), Diana DeGette (D-CO) and Betty Sutton (D-OH) released last week a discussion draft of the Food Safety Enhancement Act of 2009.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...

This week the US Food and Drug Administration released the final version of Guidance for Industry: Providing Regulatory Submissions in Electronic Format-Drug Establishment Registration and Drug Listing.

In two whistleblower suits filed in May 2009, the United States and 16 states alleged that Wyeth (Madison, NJ) knowingly failed to offer the government the same discounts it gave to private purchasers of its drugs, as Medicaid laws require.