
The company is investing $130 million to add Phase III through commercial-scale manufacturing suites to its gene therapy campus in Harmans, MD.

The company is investing $130 million to add Phase III through commercial-scale manufacturing suites to its gene therapy campus in Harmans, MD.

The new facility will be adjacent to Thermo Fisher’s newly expanded biologics manufacturing facility.

The new line has the ability to aseptically fill powder, liquid, suspension, and combination forms into vials in clinical or commercial batch sizes.

AMRI will provide AstraZeneca with manufacturing capacity and sterile fill/finish services at its drug product manufacturing facility in Albuquerque, NM.

As public confidence in the drug development process waivers, leading vaccine developers promise to adhere to scientific and regulatory principles.

The companies have signed a partnership agreement to provide extractables and leachables testing at the SG US Technology Excellence Center in Boston, MA.

The new line will add capacity to Catalent’s pipeline of clinical programs and commercial launches at the site.

Alcami will provide services for Trevena’s OLINVYK (oliceridine) injection, an opioid approved for the management of acute pain severe enough to require an intravenous opioid analgesic in adults.

BIO leaders urge biopharmaceutical companies to apply scientific principles in seeking drug and vaccine approvals.

Rapid scale-up to billions of doses requires collaborative, all-out efforts by innovators, their manufacturing partners, and the entire supply chain.

Risk-based decision-making is impacting all aspects of manufacturing quality from raw material supply to facility inspections.
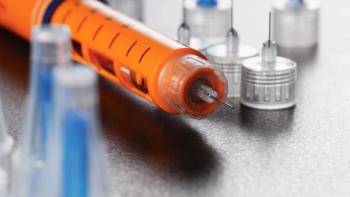
A device manufacturing process must be carefully designed in the early stages of development to ensure success in commercial manufacturing.

The general principle of lyophilization has hardly changed, but significant advances have occurred in process and product attribute understanding.

Despite pharmacovigilance legislation being in place for nearly a decade, many companies are still struggling to fulfill obligations.

Amid high expectations for a vaccine, bio/pharma readies capacity, weighs pressures.

The companies have entered into a strategic partnership for the CMC development and manufacturing of Ansun’s biologics pipeline.

The companies will collaborate on the production of a novel anti-SARS-CoV-2 immunoadhesin in iBio’s FastPharming manufacturing system.

Supply chain traceability is essential in the manufacture of APIs to assure safety and quality.

Dimethyl sulfoxide is increasingly used in high-risk parenteral and medical device applications that must be manufactured as sterile products in their finished form. A study evaluated the effects four sterilization techniques have on the product quality of this ingredient.

Prior to use in a continuous manufacturing system for oral solid dosage forms, loss-in-weight feeders need to be tested and validated to understand the performance capabilities of a given material–feeder combination. In this article, the proper strategy for set-up and optimization of a loss-in-weight feeder is demonstrated for a range of materials. The optimized set-up of the feeders was demonstrated to provide suitable performance for even the most challenging, poorly flowing materials.
Early adopters and equipment manufacturers refine their equipment and processes, paving the way for broader use.

For certain APIs, including semi-synthetic antibiotics, continuous filtration and drying improves productivity and quality.

Increased use of nested and ready-to-use primary packaging has resulted in a need for more validation data on container-closure integrity. This article describes efforts to develop these data for new and traditional containers and closures.

Negotiations for a final advance purchase agreement where the Government of Canada purchases the vaccine candidate on a not-for-profit basis for emergency pandemic use are underway.

The vaccine will be supplied by Moderna and distributed in Japan by Takeda Pharmaceutical starting in the first half of 2021 if the vaccine candidate receives regulatory approval.