
As the facility becomes fully operational, the company believes the potential risk of a shortage of the product due to increasing demand will be significantly reduced.

As the facility becomes fully operational, the company believes the potential risk of a shortage of the product due to increasing demand will be significantly reduced.

The fully integrated, pre-qualified, pre-installed aseptic filling facility can be shipped globally for drug development and the manufacturing of personalized medicines and clinical trial supplies.

The facility is now equipped to handle commercial manufacturing of a sterile injectable product in a pre-filled syringe presentation.

The agreement centers around the development of new stem-cell derived allogeneic T-cell therapies for the treatment of cancer.

GE Healthcare Life Sciences’ new facility for cell and gene processing supplies will be open in 2022.

A development and manufacturing partnership with Thermo will allow Civica to expand its internal pipeline of medications for use in emergency and critical care in the United States, further preventing the risk of drug shortages.

The formation of the new gene therapy company stems from the progress and success of Nationwide Children’s Hospital’s clinical manufacturing and gene therapy work.

The next-generation gene editing system can be applied to the development of novel cell and gene therapies.
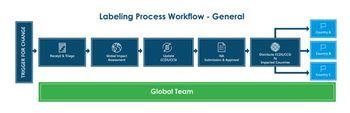
Tracking and implementing label changes are crucial to the lifecycle of a marketed drug product.

Achieving effective manufacturing processes and sufficient capacity remains a top priority across a diversified biologic drug pipeline.

As mergers continue and operations become more complex, simplifying procedures and training can prevent costly morale, quality, and compliance problems.

ProBioGen and Lava Therapeutics have closed the cell line development and manufacturing agreement for Lava’s novel bispecific antibody lead candidate.

Catalent’s FlexDirect service offers sponsors multiple delivery options from a single inventory.

The therapy is currently approved in the EU as a gene therapy for the treatment of patients 12 years and older with transfusion-dependent β-thalassemia.

The trial will test experimental stem-cell treatments against biologic therapies for severe forms of relapsing multiple sclerosis.

Phillips-Medisize has announced that it is expanding its current Global Innovation and Development (GID) site in Struer, Denmark, to include a dedicated manufacturing development and clinical build unit.

The Ireland-based company can now offer complete elemental impurities testing services.

Molecular taggants from Applied DNA Sciences and Colorcon’s Opadry coating target counterfeit and falsified medications.

The new site can manufacture oligonucleotide APIs at large scale.

With a positive employment market, some bio/pharma professionals explore options for career advancement.

Data collected through the Industrial Internet of Things enable predictive maintenance.

Advanced dynamic process control of a fluid-bed granulation process using PAT data improves product quality.

Pharmaceutical companies work toward a circular economy by using sustainable packaging.
Regulatory mandates, niche diseases, and patient-centric solutions have all impacted pharma packaging over the years and are expected to help shape the future of the sector.

PTE looks ahead to 2020’s edition of Pharmapack-the pharma industry’s dedicated packaging and drug delivery event.