
Nanoform has unveiled its plans to construct a GMP manufacturing plant that will provide API ‘solution to particle’ nanoization for clinical trials

Nanoform has unveiled its plans to construct a GMP manufacturing plant that will provide API ‘solution to particle’ nanoization for clinical trials

Capital Recovery Group, Federal Equipment Company, Heritage Global Partners, and PPL Group purchased a 110-acre campus with a flexible pharmaceutical manufacturing and packaging facility from a generics manufacturer in Huntsville, AL.

A new facility in California will expand Orchard Therapeutic’s capacity to develop and deliver lentiviral vector and gene-corrected hematopoetic stem cells.

TrendMiner 2018.R2 software for analysis of time-series data includes the ContextHub platform for expanded, self-service analytics capabilities.

AqVida, winner of the 2018 CPhI Excellence in Pharma Award for manufacturing technology and equipment, identifies advantages of robotics compared to conventional automation in fill/finish operations.

The SafeGuard Preventative Maintenance Solution from PSG’s Griswold provides remote monitoring for a centrifugal pump and its motor.
The Gold Cone X-Flo Filter Cartridge from Camfil APC stays cleaner and lasts longer than conventional pleated filters.

The company is investing approximately $14 million to expand biologics packaging capabilities and capacity at its biologics manufacturing facility in Bloomington, IN.

The company will use GE Healthcare’s off-the-shelf KUBio biologics factory, which is expected to start operations in 2020, to provide development and manufacturing for early- to late-clinical and early-commercial manufacturing stages.
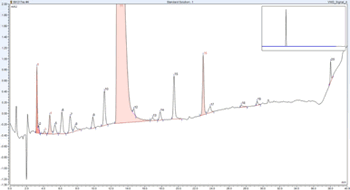
Integration of two separate chromatography data systems boosts workflow efficiency.

Boehringer Ingelheim plans to develop and test new strategies at its Solids Launch facility.

Transdermal patch design, materials, and manufacturing variables, as well as drug formulation and interactions between the API and the adhesive, can affect adhesion and drug delivery.

Virpax’s Patch-in-a-Can technology delivers pain medication using a metered-dose spray film.

To improve production in pharmaceutical manufacturing, the IT, OT, and Quality functional groups must work together to get the most value from existing plant data.

Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.
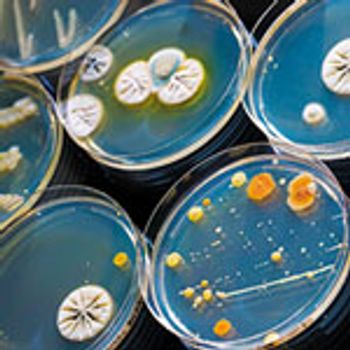
Microbial identity data can be critical for determining contamination sources.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business goals.

Once described as “throwing processes over the wall,” tech transfer is evolving into close collaboration and communication, as potential problems are considered sooner, and new technology is applied. Joseph Szczesiul, director of technical services for UPM Pharmaceuticals, shares best practices.

Charles Ross & Son Company recently developed two specialty customized 150-gallon double planetary mixers (Model DPM-150) with patented high viscosity blades.

Adents' DispaX offers improved supply-chain traceability for pharmaceutical warehouses, wholesalers, distributors, and dispensers such as pharmacies and hospitals.

Owner of Immuno Biotech, David Noakes, has been sentenced to 15 months in prison over charges of manufacturing, selling, and supplying an unlicensed medicine, as well as money laundering.

In a strategic move to strengthen its core life science businesses, Bayer announces 900 R&D and 350 manufacturing job cuts.

Contract development and manufacturing organization (CDMO), Yposkesi, has entered into an agreement in principle with Axovant Sciences.

Five additional European Union member states have been confirmed by the US Food and Drug Administration (FDA) as capable of performing good manufacturing practice inspections at a level equivalent to that of the United Sates

The investment builds on a collaboration the companies entered into in 2007 for various biomanufacturing projects.