
The author presents best practices for extractables and leachables.

The author presents best practices for extractables and leachables.

Solid-dosage forms and parenteral products benefit from next-generation packaging machines.

A screening method predicts delamination in primary packaging.
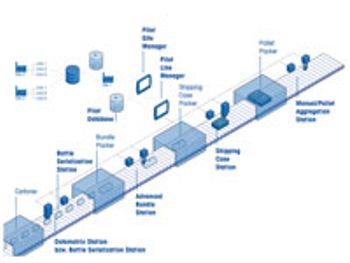
Integrated serialization systems keep pace with industry demand.

Increasing production efficiency while ensuring high parenteral product quality is the goal of both manual and automated visual inspection systems.

An annual Healthcare Compliance Packaging Council competition recognizes innovative pharmaceutical packages designed to improve patient adherence.

The annual INTERPHEX show presents end-to-end packaging solutions.

Bristol-Myers Squibb embarks on a multi-year journey to overcome the challenges of serialization and reap the benefits.

Room-temperature sterilization using nitrogen dioxide gas benefits parenteral drugs.

Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.

Medication safety and efficacy depend on maintaining products at the proper temperature.

Protecting patients from counterfeit medicines is a pressing issue facing governments and the pharmaceutical industry.

Advances in data loggers and radio-frequency identification tags help meet the increasing need for managing the pharmaceutical cold chain.

Plastic is finding increased use in vials and syringes as concerns about glass breakage and delamination and desire for increased functionality lead pharmaceutical companies to consider alternatives.

As the battle against counterfeits gains momentum, PharmTech speaks to Mark Davison, CEO of Blue Sphere Health, and Craig Stobie, global life sciences sector manager at Domino Printing Sciences, about the challenges involved.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

Overt and covert packaging technologies have evolved to authenticate drugs and fight counterfeits.

Even when all is well at the facility, one must expect the worst while braving the elements.

Overt and covert packaging technologies evolve to authenticate drugs and fight counterfeits.

New US Pharmacopeial Convention standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.

Only the strong survive when it comes to pharmaceutical packaging and shipping.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the September 2012 edition from Uhlmann Packaging Systems and GE Healthcare.

Packaging and monitoring tools protect temperature-sensitive pharmaceuticals.

International trade can be great for business, but breaking border laws can put one in hot water.

Highly automated and sensitive quality-control equipment quickly identifies product faults.