
MG America's ACE-BT300 Coding and Verification Unit provides track-and-trace capabilities for bottles.

MG America's ACE-BT300 Coding and Verification Unit provides track-and-trace capabilities for bottles.

The FXS Combi from Bosch Packaging Technology features an integrated capping station for vials and cartridges.

The Press Out Universal Mini from Sepha can deblister at a speed of up to 15 packs per minute.

The Manesty TPR 500 tablet press from Bosch Packaging Technology increases output and features a hygienic design.

Tony Wright, CEO of Exelsius Cold Chain Management, answers questions about the new requirements for temperature-controlled pharmaceutical distribution.

New injection-delivery systems with multiple closure points pose challenges for container closure integrity testing.

Displays will include new products for anticounterfeiting, labeling, and compliance packaging and new equipment for filling, aggregating, and quality control.

FDA clarifies recommendations for injectable drug products packaged in vials and ampules.

Manufacturers are taking measures to comply with new package safety rules.

Using best practices for manual or automatic inspection can improve the inspection process.

Trends driving pharmaceutical packaging include product protection, productivity boosters, and patient adherence improvement.

The Intelli-Code Carton Coding and Inspection System connects to serialization data-management systems.
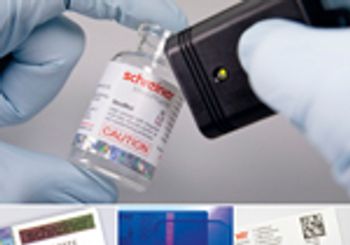
A combination of overt and covert features protects against counterfeiting.

Manufacturers should plan ahead when implementing a serialization project to comply with the Falsified Medicines Directive.

A packaging defect has led to Merck voluntarily recalling all lots of Liptruzet tablets in the United States.

PCI discusses the challenges and benefits of serialization in light of the new US track-and-trace legislation.

Kurt Lumden, Director, Client services at PAREXEL's Perceptive Informatics, discusses the management of investigational product temperature excursions.

USP is developing and revising distribution standards in response to changes in the global supply chain.

Standards for ferrules and cap overseals focus on explicit warnings.

The Drug Quality and Security Act creates national standards for serialization of drug products to protect against counterfeiting.

Capacity expansions and new products meet needs for inhaler and injector systems.

Sepha launches BottleScan, a multi-bottle integrity tester for induction-sealed pharmaceutical bottles.

Temperature-sensitive pharmaceuticals and biologics depend on a variety of services and technologies to establish, maintain, and verify proper storage and transport conditions.

USP seeks input from stakeholders on new and revised standards to mitigate extractables and leachables in plastic packaging systems.
New weapons in the anticounterfeiting arsenal include authentication and labeling technologies.