
CDMO Vetter announced the addition of a new flexible serialization service, introduced in response to stricter packaging regulations calling for drugs to be serialized as a means to fight counterfeits.

CDMO Vetter announced the addition of a new flexible serialization service, introduced in response to stricter packaging regulations calling for drugs to be serialized as a means to fight counterfeits.

The Baxter recall in the US of one lot of highly concentrated potassium chloride is due to a mislabeled overpouch.

New child-resistant packaging designs meet regulatory requirements, and consumer research into child-resistant closures continues.

Cryopreserved shipments are monitored with real-time tracking and intervention services.

Contract Packaging Resources issued a voluntary recall of naproxen sodium tablets because some cartons contain bottles of ibuprofen.

Packaging machines, coding equipment, inspection and data capture systems, material handling, controls, and software must work together in a serialization system.

The intermittent cartoner can be equipped with a variety of in-feed systems and can handle different package formats and closure options.

Monitoring device uses thermal imaging to inspect 100% of sachets and pouches.

Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.
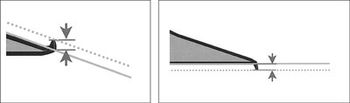
To achieve the high quality standards required for critical defects in pharmaceutical glass syringes, a combination of visual and camera-based inspection technologies are used.

InnoPack, which runs alongside CPhI Worldwide in Paris Nord Villepinte, France, is specially focused on pharmaceutical packaging. This is the place where companies gather to showcase their latest innovations and technologies in packaging. In this article, Martin Dallas from Essentra explains the role of innovative packaging technologies.

GS1 publishes a healthcare industry guideline describing how to implement GS1 standards to support requirements of the 2013 US Drug Supply Chain Security Act.

The EdgeTRAC software Print, Apply, and Verify module from ROC IT Solutions can be used to implement serialization in manual packing operations.

Use of temperature-controlled packaging is increasing with the growing need for cold-chain protection.

A semi-automatic case packer from Omega Design and Brazil's Grupo Tecnor offers unit-level serialization capabilities.

Kurt Lumsden, Director, eCDS Client Services at PAREXEL Informatics, discusses the use of interactive response technologies in investigational product expiry management.

The pharmaceutical industry has traditionally used glass as a primary material for containment systems due to a variety of characteristics that enable generally safe and efficient drug storage. However, there are many risks associated with glass.

Travtec, a packaging line solutions specialist, has opened a second facility close to its Leigh, Lancashire headquarters. This move is part of an investment to expand the company?s operations to meet continuing demand for its systems, particularly as the pharmaceutical industry prepares for the current and forthcoming track and trace legislation, including the 2017 European Falsified Medicines Directive.

PharmaChk, a portable device being developed to detect substandard medicines, receives $2-million grant.

The US federal Drug Quality and Security Act (DQSA) and more specifically the Drug Supply Chain Security Act (DSCSA), referring to Title II of the law, requires phase-in of requirements to prevent counterfeiting. An expert discusses how companies should be preparing to meet the requirements of the DSCSA.

Pharmaceutical manufacturers will be-and are already&mdashfacing enormous challenges to implement the serialization regulations within the given timeframe.

In the past 10 years, safety issues have become increasingly important in pharmaceutical packaging, from needle sticking safety to counterfeit protection.

Packaging Coordinators, Inc. expands its services with acquisition of Penn Pharmaceutical Services.

Modular design and quick-changeover features help deliver flexibility and maximize return on investment.

West's new plant in India will manufacture primary packaging for injectable drugs.