
EMA and FDA publish joint QbD guidance on design space verification.

EMA and FDA publish joint QbD guidance on design space verification.

Zhoydro ER is the first drug to have updated labeling now required for all ER/LA opioid analgesics.

The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs.

FDA is seeking a permanent injunction against a dietary supplement manufacturer following the company?s repeated distribution of unapproved drugs and adulterated dietary supplements.

During the ongoing federal government shutdown, FDA activities will be limited to work involving the safety of human life or the protection of property, and activities funded by carryover user fee balances PDUFA, GDUFA, and MDUFA.
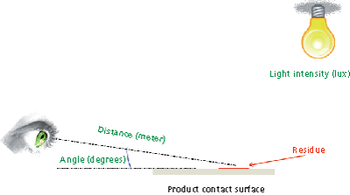
The authors evaluated a variety of materials of construction (MOCs) and found that visible residue limits (VRLs) were higher on some MOCs than on stainless steel. The optimal viewing conditions were dependent on the MOC and the viewing background. The risk of a cleaning failure due to visual failure for different MOCs can be mitigated or eliminated using complementary cleaning validation studies.

Shamrock Medical Solutions Group, a drug repackaging and distribution company, repeatedly failed to comply with good manufacturing practices.
The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs
Case studies on the manufacture of a bluk powder and the development of a tablet show the application of QbD principles.

Installation of a quaternary chiral center with high enantioselectivity using memory of chirality enabled the six-step synthesis of the desired active compound.

Agencies collaborate to ensure consistent product quality.

CDER withdraws some outdated guidance documents and makes plans to finalize others.

Successful product development and technology transfer between a CMO and the sponsor company requires a multifaceted collaborative approach. The author, Marga Vines from Grifols International, analyzes a project for an injectable drug for which a new drug application was submitted to FDA.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Until recently, glycan analysis has been a slow, labor-intensive process more widely used late in bioprocess development. New high-throughput methods are changing that.

ISPE study reveals quality systems of manufacturing as the leading cause of drug shortages.

The company is cited for using unapproved visual-inspection methods for finished parenteral drugs and conducting inadequate visual inspections.

Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products made from its facility in Ingelheim am Rhein, Germany.

An introduction to the upcoming Interphex panel--Lessons Leaned: Successes and Challenges in Implementing Quality by Design.? Moderator: Jennifer Markarian, manufacturing editor, Pharmaceutical Technology. Panelists: John Lepore, senior director of Chemical Process Development and Commercialization for Global Pharmaceutical Commercialization at Merck and Co. Chris Moreton, FinnBrit Consulting Jonathon Thompson, senior manager of Compliance Services Consulting at Invensys Operations Management

Prefilled-syringe line features automation and novel disinfection techniques.

The European Union authorities are stepping up their efforts to incorporate quality-by-design principles into their regulations and guidelines.

Keith Bader, senior director of technology at Hyde Engineering + Consulting discusses his presentation ?Establishing a design space: cleaning process development and validation,? which will be presented at Interphex 2013

Victor Hernandez of EMD Millipore discusses his upcoming session, "Current continuous process validation program: following FDA current guidelines,? which will be held at Interphex 2013
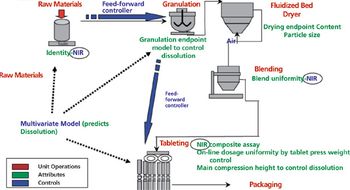
The authors present topics discussed and conclusions that resulted from the PDA QbD workshop.

Factors for assessing excipient variability, the associated challenges developers need to address to design and manufacture solid oral drug products, and solutions for such challenges are examined.