
Visiting a new site or going down memory lane may not get you where you want to go.

Visiting a new site or going down memory lane may not get you where you want to go.

Quality management requires more effort in a complex supply chain.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.

The authors share their approach and experience working in complex, multicompany environments for in-licensed products to develop successful chemistry, manufacturing, and controls packages for managing outsourcing partnerships.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.
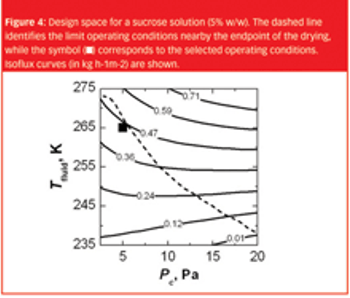
The same recipe obtained in laboratory-scale equipment cannot, without modifications, generally be used to freeze-dry the product in a pilot-scale or industrial-scale freeze-dryer.

Getting an answer is easy-asking the right question is apparently more difficult.

Sometimes, there are just too many cooks in the kitchen.

Many factors affect research results.

In any industry, inspections can be a pain, and pharma is no exception.

The need for greater process understanding raises the bar for suppliers.
The author provides an overview of QbD implmentation for biopharmaceuticals.

Just when things seem to be looking up, the unexpected problem occurs.

Taking care to note, file and re-check information can save one from future mishaps.

The authors highlight the need for a technology-transfer process that is efficient, cost-effective, and repeatable, stressing the importance of process understanding. Read this and other preferred organization articles in this special issue.

FDA published its long-awaited guidance titled Process Validation: General Principles and Practices this week.

A company's contamination-control plan is an important document designed to formalize the rationale, methods, and validation of contamination-control procedures in a manufacturing facility. The author describes the role of bioburden in the contamination-control plan.

Sometimes doing what you think is right ends up being completely and utterly wrong.

Remaining calm, cool, and collected during mergers and inspections is a feat in itself.

This article examines the difficulties in designing lyophilisation processes that can be faithfully scaled up to production volumes and suggests the most effective ways in which this can be achieved.

From disagreement to denial, being cordial about quality control can be challenging.

The authors present three approaches that a contract development and manufacturing organization can consider when designing development and process-optimization studies that will provide useful data for scaling up a project.

From weekend deliveries to nonsterile gloves, a single exception can make a product fall flat.

The authors discuss a continuous-flow reactor that avoids parallel channels and enables economic plant setup. This article is part of a special issue on API Development, Formulation, Synthesis and Manufacturing.

Cases of overlooking proper packaging, reconstitution, directions, and dissolution.