
The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

If you’ve worked in the drug industry for a while, chances are good that you remember Pfizer’s Rezulin, which was produced through hot melt extrusion (HME). If you work for a major pharmaceutical manufacturer, it’s likely that your company owns a twin-screw extruder. Yet HME has not been a common way of manufacturing drugs, and many industry employees don’t understand the principles of HME or the advantages that the technique offers.

Laboratory personnel share interesting tales as well as stories of unexpected tails.

Continuous manufacturing could bring the pharmaceutical industry advantages such as improved product quality, easy scale-up, flexibility, and short cycle times. But how can a company switch from batch to continuous manufacturing?

From last-minute product inserts to putting out fires, close calls are a common occurrence.

Sharing too much-or too little-information can have disastrous onsequences.
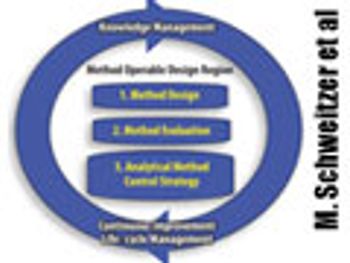
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods. This article contain bonus online material.

The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods.

References for the article published in the February issue of Pharmaceutical Technology Europe.

Could flexible manufacturing change the standards for biopharmaceutical production? To find out, Equipment and Processing Report talked to James Robinson, vice-president of technical and quality operations at biotechnology company Novavax (Rockville, MD).
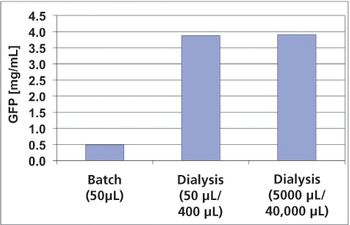
The authors sought to improve the productivity of protein synthesis by using a highly active cell-free extract from Escherichia coli and by optimizing buffer conditions and shaking conditions.

Directors and staff miss the mark when it comes to following procedures.

Blame it on cafeteria gossip, outdated procedures, and major miscommunication.

Recent advances in microreactor technology are improving the application and scale at which the technology can be applied.

New tests solve one issue, but cheaper plastic and new stoppers cause problems.

The manufacturing process, which influences a drug's safety and efficacy, is particularly critical for drugs administered through injection, and personnel must closely supervise lyophilization to ensure product quality.

Reading PharmTech's October article on Critical Challenges to Implementing QbD gave me the impression that we might have the road map to Moksha or Nirvana. Unfortunately that was not meant to be.

The authors relay the outcome of a two-day workshop that brought together regulators and generic-drug industry representatives.

Like life, the workplace also can have many surprises.

Representatives of one pilot program participant, Wyeth, outline the experiences and lessons learned for implementing a science- and risk-based approach to drug-development and manufacturing.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Officials from the US Food and Drug Administration discuss best practices for applying quality-by-design concepts. This article contains bonus online-exclusive material.

A review of NIPTE's core projects and its plans for training-and retraining-the pharmaceutical industry.

The author describes the framework needed to implement QbD and achieve the deeper process understanding that is fundamental to QbD.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.