
Understanding how to identify, remediate, and prevent facility infection is crucial for product quality.

Understanding how to identify, remediate, and prevent facility infection is crucial for product quality.

Meggle Group Wasserburg has been granted an EXCiPACT certificate.
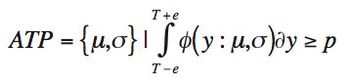
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

With a quality-by-design approach, robust processes consistently can help deliver quality product.

FDA found violations of cGMP at finished-drug manufacturer in India.

Quality by design, process analytical technology, and high throughput screening have helped drive progress in pharmaceutical development.

Quality by Design has initiated a paradigm shift in solid dose pharmaceutical manufacturing.

Contract service providers describe how quality by design has influenced a drug sponsor's expectations of suppliers.

Baxter investigates root cause of cellulosic fibers and/or plastics in four lots of IV solutions.

Sun Pharma, Forest Pharmaceuticals and West-Ward Pharmaceuticals issue recalls over dissolution issues.

Baxter Healthcare has initiated a nationwide recall of more than 20,000 containers of a pre-mixed beta blocker due to the presence of particulate matter.
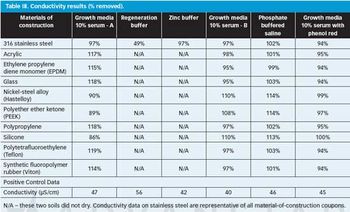
The authors look at the cleanability of pharmaceutical soils from a variety of materials of construction to determine the relative ease of cleaning and explore potential grouping strategies as part of a comprehensive cleaning validation program.

Bristol-Myers Squibb issues a voluntary US recall of Coumadin (warfarin sodium) for injection due to presence of particulate matter.

GSK Biologicals receives warning about cGMP compliance issues at its Quebec facility.

FDA cites cGMP violations for API manufacturing at a facility in Tianjin, China.

FDA inspection found cGMP violations in the Bangalore, India API manufacturing facility.

ISPE Metrics Pilot Program is designed to demonstrate the feasibility and value of standard quality metrics.

Despite GMP deficiencies, EMA reinstates GMP certificate for Ranbaxy's Toansa facility, citing no threat to public health.

Hospira has recalled one lot of labetalol hydrochloride injection, USP, due to visible particulates.

Laser Diffraction Software Supports Analytical QbD

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

Agencies extend successful pilot program to further harmonization of QbD topics.

Daiichi Sankyo announced suspension of API shipments from Ranbaxy's Toansa and Dewas plants; issues apology to stakeholders.
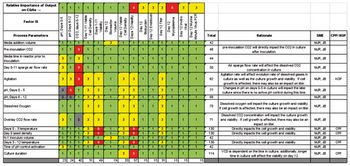
Traditional project decision-making is compared with a QbD approach.

FDA adds Ranbaxy's Toansa, India facility to existing consent decree, prohibiting distribution of APIs from that location