
US support of regenerative medicine is essential for maintaining a lead on healthcare innovation.

US support of regenerative medicine is essential for maintaining a lead on healthcare innovation.
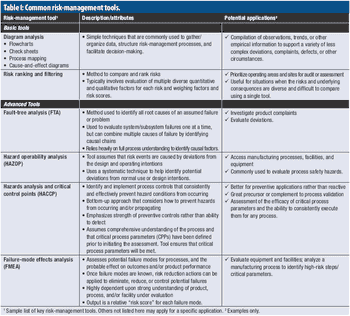
A PQRI expert working group provides case study examples of risk-management applications.

Which route will we take to arrive at a national stem-cell policy?

FDA Publishes Final Guidance on Dissolution Testing.

President Obama unveiled an Advanced Manufacturing Partnership designed to reinvigorate the country's manufacturing sector.

The European Medicines Agency (EMA) has held a second forum regarding the implementation of new pharmacovigilance legislation, which gave stakeholders the opportunity to discuss their expectations on various aspects of the new legislation's execution.

The Association of the British Pharmaceutical Industry (ABPI) has published a guidance that suggests best practices for managing adverse events and other pharmacovigilance data from the internet and social media tools.

FDA Issues Warning Letter to Dr. Reddy's Following Inspection of the Company's Mexico-Based API Manufacturing Plant.

The traditional method of conveying information in the brief summary of a printed prescription-drug advertisement is neither the most comprehensible nor the most preferred by consumers, according to an FDA study.

FDA released a new strategy on that is aimed at meeting the challenges posed by rapidly rising imports of FDA-regulated products and the growing complexity of the pharmaceutical supply chain.


FDA Issues Consent Decree of Condemnation, Forfeiture, and Permanent injunction Against H&P Industries, the Triad Group, and Three Individuals.

An FDA guidance has provided information for products that involve the application of nanotechnology, and rationales for those points.

After looking back at the first year of its Bad Ad outreach program, FDA judged that the initiative has successfully raised awareness about misleading promotion, according to an FDA press release.
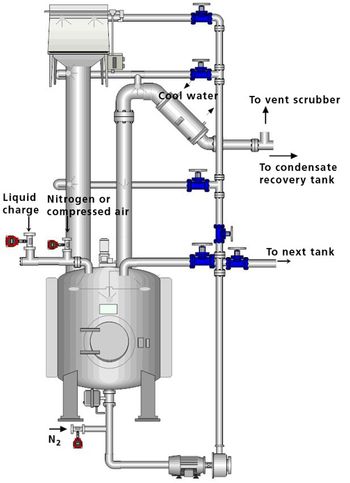
The author describes the benefits and challenges inherent to cleaning in place (CIP). The article also describes the development and validation of a CIP cycle.

AstraZeneca agreed to settle a sex-discrimination lawsuit by paying $250,000 to 124 women who worked at the company's Philadelphia Business Center.

The US Supreme Court ruled in favor of Roche in a patent-dispute case the pharmaceutical company had with Stanford University in a 7-2 vote.

The European Public Health Alliance has called for greater pricing transparency, as well as the formation of a public website that provides comparative information on medicines' procurement prices.

FDA Issues Final Guidance to Amend IND Reporting Requirements.

Ensuring compliance though increased statistical knowledge and resources.

An independent report released by the European Medicines Agency highlighted a number of recommendations to aid the agency in its communication of the benefits and risks of medicines.

Why SOPs are rarely followed, often cited, and in need of follow-through.
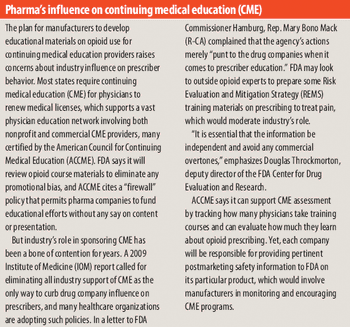
Industry struggles to curb drug abuse, diversion, and disruptions in supply.

Sometimes, there are just too many cooks in the kitchen.

FDA added a searchable database of inspection data to its website. The database lists the names and addresses of facilities that the agency inspected during fiscal years 2009 and 2010.