
A book about developing quality-control training manuals provides a wealth of information and a dearth of practical help.

A book about developing quality-control training manuals provides a wealth of information and a dearth of practical help.
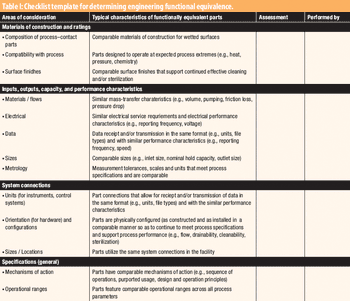
The second in a series of eight case studies from the Product Quality Research Institute focuses on functional equivalence for equipment.

Visiting a new site or going down memory lane may not get you where you want to go.

Many child-safe package designs help improve compliance and provide tamper evidence.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.

Quality management requires more effort in a complex supply chain.

Thomas P. Layloff describes the advantages of using thin-layer chromatography methods for counterfeit detection. This article contains bonus online material.

USP promotes horizontal standards for biologics' quality attributes.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.

Supplier differentiation is increasingly important in the highly competitive arena of pharmaceutical outsourcing.

Companies know they need regulatory information management, but they don't always know where to start and how to weave this capability across diverse technology applications.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.

Avantor reviews its experience with the Rx-360 shared audit pilot program, which is aimed at protecting the pharmaceutical supply chain. This article is part of a special issue on Outsourcing.

The global production of seasonal influenza vaccine will double to 1.7 billion doses by 2015, according to a World Health Organization presentation.

A public consultation has opened in Europe for the revised guideline on the good distribution practice of medicinal products for human use.

FDA issued a draft guidance for industry comment on proposed policy for diagnostic tests used with targeted drug therapies, that is personalized medicine.

FDA Approves the Influenza Vaccine Formulation for the 2011-2012 Flu Season.

The pharmaceutical industry and US regulatory bodies have not responded adequately to the increasing level of outsourced manufacturing in countries such as China and India, according to a new white paper by the PEW Health Group.

Even though manufacturers are responsible for ensuring the quality of combination products, some companies may not be certain about what quality system to apply to their production.
![Rivera-Fig-4-new[2]web-731308-1408618780125.jpg](https://cdn.sanity.io/images/0vv8moc6/pharmtech/c5b3bd34e31edbde4e6a56be25a83c3d7a998f32-504x275.jpg?w=350&fit=crop&auto=format)
The author describes the components that make up a clean-in-place system and how the system should be built to ensure efficiency.

We?re attempting to validate a clean-in-place process using total organic carbon (TOC) as the analytical method. Our swab-sample recovery tests are giving us inconsistent results. Recovery values range from 95% to 205%, and there seems to be no correlation with the amount of drug product deposited on the coupon. What could be the problem?

FDA banned the importation of products manufactured at the Mexican unit of Dr. Reddy's Laboratories. The import ban is a result of the company's failure to correct the violations listed in a recent Warning Letter to the agency's satisfaction.

FDA issued a draft guidance, entitled "Q11 Development and Manufacture of Drug Substances," which is now available for comment according to a notice published on June 29, 2011, in the Federal Register.

The European Medicines Agency has responded positively to a new directive published in the Official Journal of the European Union addressing concerns over increases in falsified medicines in the supply chain.

An FDA panel has voted unanimously to withdraw approval for Roche's Avastin for use in the treatment of metastatic breast cancer because there is not enough data to support that the benefits outweigh the risks.