The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

EMA Hosts Subgroup Analysis Workshop.

Until now, the industry has adhered to the tradition of producing three batches of product to validate its manufacturing processes. But FDA?s new process-validation guidance does not prescribe any number of batches that is necessary for compliance.

EMA Issues Concept Paper for Public Consultation on Development of Toxicological Guidance for use in Risk Identification.

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.

Careful mixing during a product's distillation can help avert trouble from a strong concoction.

Russia is aiming to provide an alternative to China and India for drug manufacturing, including APIs.

June and July saw three major US conferences on implementing single-use technologies: the IBC Single-use Applications meeting, the PDA Single-use Workshop and the Bio-Process Systems Alliance (BPSA) International Single-use Summit (ISUS). Jerold Martin highlights some of the key topics discussed at these meetings.

In an age dominated by the internet and uncertainty over the best packaging security methods to employ, counterfeit medicines have the ideal environment to thrive.

Last week, FDA published a final guidance for pharmaceutical manufacturers that plan to incorporate physical–chemical identifiers in solid oral dosage forms as an anticounterfeiting strategy.

Letting contamination build up can cause multiple headaches.

In an effort to balance bilateral trade, India is urging China to increase Indian pharmaceutical imports.

Manufacturers fund research and reduce prices to tackle diseases.

FDA has published a guidance on Marketed Unapproved Drugs-Compliance Policy Guide, which describes the agency's enforcement priorities with regard to products that lack regulatory approval or that are not marketed in accordance with the over-the-counter drug review.

FDA has issued a guidance titled User Fee Waivers, Reductions and Refunds for Drug and Biological Products that outlines FDA's policies for issuing waivers, refunds or reductions in prescription drug user fees.

USP Expands Presence in India.

FDA's Division of Drug Marketing, Advertising, and Communication issued a letter to Pfizer's vice-president of US Regulatory Affairs regarding its online resources page for Lipitor tablets.
PDUFA renewal legislation sets stage for new policies affecting revenue, resarch, and oversight.

Being aware of a forthcoming inspection or how a product was made can make a huge difference.
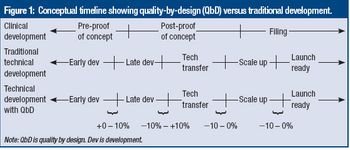
Directors from FDA's Center for Drug Evaluation and Research summarize findings in an FDA-commissioned report on QbD and propose actions the agency can take to encourage full-scale QbD implementation.

Growth and change in Brazil and Mexico offer key opportunities for the region's pharmaceutical industry.
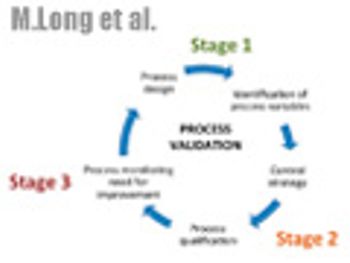
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

USP and the Brazilian National Agency of Sanitary Surveillance are teaming up to develop joint education activities for professionals involved in formulating and using pharmacopeial monograph standards in Brazil.

FDA published a draft guidance that lists recommendations to follow, data to provide, and criteria to meet and describe in new drug applications and abbreviated new drug applications for scored tablets.

EMA announced that a new version of the validation criteria for electronic applications for human medicines comes into effect on Sept. 1, 2011.