
EMA's revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

EMA's revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

FDA cites cGMP violations of finished pharmaceuticals at the company's facilities in Marion North Carolina and Jayuya, Puerto Rico.

The company is cited for using unapproved visual-inspection methods for finished parenteral drugs and conducting inadequate visual inspections.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.

A unique demographic and payer mix make ASEAN an increasingly attractive region.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

The Taiwanese government engages in regulatory science in a move to boost its pharmaceutical sector.

An overview of the latest regulatory developments for malaria drugs, biosimilars, and global standards.

Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products made from its facility in Ingelheim am Rhein, Germany.

The draft guidance describes how quality agreements can be used to delineate the responsibilities of contract manufacturers involved in the cGMP manufacture of APIs and finished drug products.

FDA has released guidance on best practices for conducting and reporting pharmacoepidemiologic safety studies.

EMA streamlines orphan drug application procedure.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

FDA faces budget crunch; Supreme Court hears key cases

The author provides a review of FDA's guidance document, Guidance for Industry: Q11 Development and Manufacture of Drug Substances, and its relation to the International Conference on Harmonization's Q11 document and its application to the industry.

FDA issues draft guidance to minimize medication errors.

FDA has released Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination.

Prefilled-syringe line features automation and novel disinfection techniques.

Ruling has implications for intellectual property protection for innovator drugs in India.

Latin America's diverse growing market seeks regulatory harmonization.
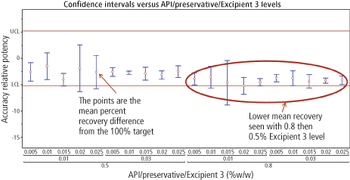
The authors describe a method-validation-by-design (MVbD) approach to validate a method over a range of formulations using both design-of-experiment and quality-by-design principles to define a design space that allows for formulation changes without revalidation.

Novartis loses an appeal in seeking patent protection in India for its anticancer drug.