
REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

Some GMP agents seem to find a way to squander time, money, and common sense.
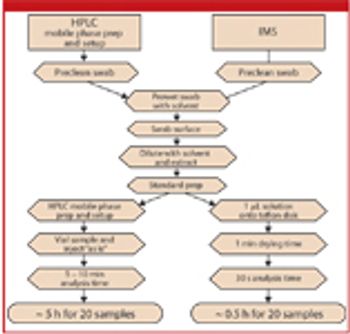
The authors discuss the theory of ion mobility spectrometry, its benefit over HPLC analysis in cleaning verification, and the experimental considerations for method validation and validation.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.
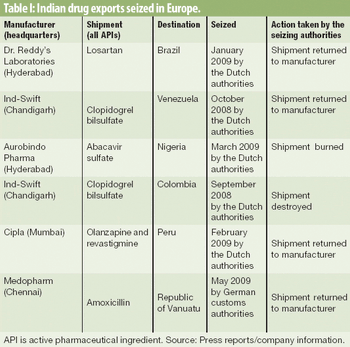
Patent infringement claims and a lack of clear global trade distribution routes may be unraveling the country's generic-drug export industry.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

The US Food and Drug Administration released a new guidance for industry, Medication Guides-Adding a Toll-Free Number for Reporting Adverse Events, on June 8, 2009.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

This week the US Food and Drug Administration released the final version of Guidance for Industry: Providing Regulatory Submissions in Electronic Format-Drug Establishment Registration and Drug Listing.

Short-term problems in software or hardware lead to long-term manufacturing troubles.
Despite its shrinking domestic economy, Ireland is determined not to let its pharmaceutical industry fade into the shadow of global recession.

Agency officials and manufacturers anticipate stricter enforcement of drug safety and quality.

The US Food and Drug Administration posted a draft guidance for industry, Presenting Risk Information in Prescription Drug and Medical Device Promotion, on its website May 26, that describes factors the agency considers when evaluating advertisements and promotional labeling for prescription drugs and medical devices.

The US Food and Drug Administration issued a draft guidance for industry, Label Comprehension Studies for Nonprescription Drug Products, in the May 1 Federal Register.

Process steps, GMP documents, a purification vessel, and validation seem to disappear.

Government funding is slated to boost comparative studies of prescription drugs.

The author describes various manufacturing processes and evaluates whether the guidance can be applied to each of them.

As the pharma industry desperately seeks to cut costs and streamline production, a new breed of CMO is growing in Europe. Find out how today's CMO is capitalizing on pharma's plant closures and divestments.

The US Food and Drug Administration finalized a Guidance for Industry this week that aims to clarify the submission of new drug applications (NDAs) and biologics license applicants (BLAs) using the common technical document (CTD) format, including the electronic CTD (eCTD).

The US Food and Drug Administration issued a draft guidance for industry on the Submission of Summary Bioequivalence Data for Abbreviated New Drug Applications (ANDAs).

The US Food and Drug Administration issued 14 untitled letters last week to pharmaceutical companies in violation of the Federal, Food, Drug, and Cosmetic Act for publishing misleading and misbranded information about their drug products online.

Although industry is tightening its belt, contract manufacturers across Europe are actually making out quite well by taking on additional projects and new roles.

FDA is poised to gain authority and resources to ensure the quality of food and drugs.

GMP agents report on old products, aseptic violations, and unexpected emotions.

On March 3, the US Food and Drug Administration released a draft guidance for Industry entitled "Clinical Pharmacology Section of Labeling for Human Prescription Drug and Biological Products: Content and Format."