
Aptar Pharma has signed a license agreement with BD to jointly develop and bring a new autoinjector to market.

This article will clarify reasonable expectations for the responsibilities of topical product formulation developers and for excipient suppliers regarding the information and samples for experiments needed for QbD.

Aptar Pharma has signed a license agreement with BD to jointly develop and bring a new autoinjector to market.

Analytics and science-based approaches are shedding more light on these complex dosage forms, promising to improve process control and product design.


Topical semi-solids, like creams, lotions and ointments are comprised of a complex mixture of microstructures or colloidal phases, frequently referred to as the Q3. These microstructures may include micelles, lamellar phases, polymer matrices, liquid crystalline and crystalline states of wax excipients, as well as the solid states of active pharmaceutical ingredients. Different topical medicated semi-solid formulations, spanning a range of treatment indications, reveal a variety of different microstructures.

The agency says the increasing requests for orphan drug designation has resulted in a change in FDA’s review goals.

The drug received breakthrough therapy and orphan drug designation as a monotherapy for the treatment of chronic graft-versus-host-disease.

The company expanded its topicals capacity with an investment in the Becomix RW30 model homogenizer.

The agency publishes draft guidance on assessing the adhesion of transdermal delivery systems and topical patches.

The agency found no differences in risk of pneumonia for different products.

Novartis announced on Jan. 5, 2016 that the company is collaborating with Qualcomm to develop cloud-based technology for COPD patients.

Ionis Pharmaceuticals receives orphan drug designation for HTTRx for the treatment of Huntington’s disease.

3M and Impel will collaborate to develop and commercialize Impel’s Precision Olfactory Delivery technology for enhanced central nervous system drug delivery.

The agency has set up a workshop on how to demonstrate the benefits of orphan drugs over existing treatments.

Current guidance for absorption of elemental impurities does not address dermal exposure, resulting in a simplistic approach to limit setting.

Successful drug delivery via a dry powder inhaler is determined by the API physicochemical properties, the formulation composition and process, the device and operating conditions, the patient–device relationship, the environmental variables, and ultimately, patient compliance.

Pharmaceutical Technology spoke with David Fitzhenry about the costs of specialty drugs.

Experts attending the European Psychiatry Association Congress in Vienna say that Adasuve has made an impact in the treatment of agitation in patients suffering from schizophrenia or biopolar I disorder.

CVS Health signed a definitive agreement to acquire Omnicare for $12.7 billion to expand its reach to assisted living and long-term care facilities.
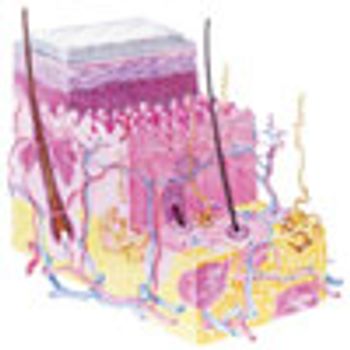
Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

Dicerna Pharmaceuticals announces that FDA granted its primary hyperoxaluria type 1 (PH1) treatment Orphan Drug designation.

Recipharm makes a strategic investment in Synthonics and partners in development of novel compounds.

Drug spending rose last year at the highest rate since 2003, driven by specialty medicines, according to a report from pharmacy benefit manager Express Scripts.

Sanofi launched a rapidly absorbed, short-acting inhalable insulin in the US to help control type 1 and type 2 diabetes.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.

Entrectinib has received FDA’s orphan drug and rare pediatric disease designations for the treatment of neuroblastoma.