
Micronization and other processes are used to obtain optimal particle characteristics for pulmonary and oral solid-dosage delivery.
Jennifer Markarian is manufacturing reporter for Pharmaceutical Technology.

Micronization and other processes are used to obtain optimal particle characteristics for pulmonary and oral solid-dosage delivery.

Flexibility, which involves the ability to quickly change product capacity or even product type to meet market demand, is increasingly important. In new construction or renovation, modular process skids and modular buildings create this flexibility. Experts discuss trends and challenges.

An expert discusses how companies should be preparing to meet the requirements of the Drug Supply Chain Security Act, which requires phase-in of requirements to prevent counterfeiting.

Manufacturers can identify and reduce waste using tools such as value-stream mapping.

The US federal Drug Quality and Security Act (DQSA) and more specifically the Drug Supply Chain Security Act (DSCSA), referring to Title II of the law, requires phase-in of requirements to prevent counterfeiting. An expert discusses how companies should be preparing to meet the requirements of the DSCSA.
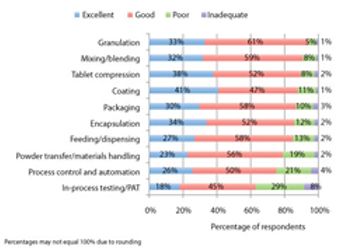
Pharmaceutical Technology’s survey collected industry feedback on trends and the utility of equipment used in finished drug-product manufacturing.
PharmTech's annual equipment and manufacturing survey shows gains in process analytical technology and indicates areas for future innovation.
Nitrogen dioxide can sterilize and depyrogenate an aseptic fill area in a blow-fill-seal process.

New advanced aseptic manufacturing technologies are available for filling liquid pharmaceuticals, including biologics.

Progress in equipment availability, process analytical technology, and advanced process control aids ongoing development of continuous solid-dosage manufacturing processes.

Pharmaceutical Technology spoke with Gloria Gadea-Lopez, associate director of Shire, and Martin Dittmer, PharmaSuite product manager at Rockwell Automation, about how manufacturing execution systems (MES) can improve productivity and the current challenges faced by companies implementing MES systems.

Experts will examine continuous processing in solid-dosage and biopharmaceutical production.
Quality-by-design principles enhance a thorough understanding of both product and process technology, which is needed for optimization of solid-dosage manufacturing, including processes for improving solubility, such as hot-melt extrusion, softgels, and liquid-filled capsules.

New methods of encapsulation and filtering address scale-up challenges.

Buying and selling used laboratory and process equipment has become mainstream; experts offer tips to potential buyers.

New facility designs enable flexible, multiproduct production.
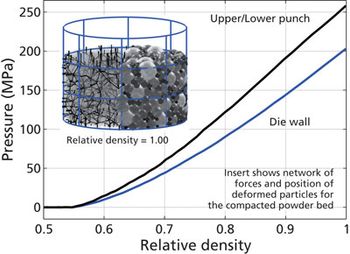
Screening methods and predictive models address tenacious tablet-sticking problems.

FDA releases a strategic plan and issues a proposed notification rule to improve prevention and resolution of drug shortage problems.

Vaporous hydrogen peroxide, nitrogen dioxide, chlorine dioxide, and carbon dioxide technologies can be used in pharmaceutical manufacturing to sterilize or disinfect.

Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.

The collaboration with Pfizer aims to develop next-generation, continuous manufacturing for solid-dosage pharmaceuticals.

Presenters at the 8th Annual Forum on Manufacturing Execution Systems (MES) discuss how a vision and common terminology help companies implement MES globally.

With the increasing use of high-potency APIs, it is more important than ever to have an appropriate containment strategy to protect both the quality of your product and the safety of your personnel.

Experts, including researchers at I Holland, Natoli, and Rutgers University’s Engineering Research Center for Structured Organic Particulate Systems, are seeking a greater understanding of the fundamental causes of tablet sticking and are developing predictive models to more quickly find solutions to specific sticking problems.

Presenters at a pharmaceutical extrusion seminar discussed formulating drugs produced using hot-melt extrusion.

Upcoming requirements in the US and around the world for serialization and track and trace of pharmaceuticals were a focus of the Pharmapack conference held in Philadelphia, PA earlier this week.

Model-predictive design is applied to solid-dosage processes.

Models and modeling software gain a foothold in solid-dosage manufacturing process design.

Making the effort to apply new methods to pharmaceutical processing will bring benefits.

Pharmaceutical Technology spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.