
Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.
Jennifer Markarian is manufacturing reporter for Pharmaceutical Technology.

Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.
Using training devices may ease patient anxiety about using autoinjectors and prefilled syringes, potentially leading to improved patient adherence.

Big data collection, advanced analytic tools, and artificial intelligence enable ongoing improvements to overall equipment effectiveness.

Industry–academia collaborations seek to address unmet needs in measurement science.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

The Global Health division of the Bill and Melinda Gates Foundation is seeking solutions for health problems, such as infectious diseases, that impact the developing world, including identifying drug delivery forms to compensate for a lack of infrastructure in these regions.

Innovative technologies, such as drug-loaded devices and 3D printing, enable advances in implantable devices and other novel dosage forms.

A twin-screw extruder can be used as a continuous wet granulator.

Engineering controls and safe practices for containment protect both operator and product in tablet and capsule production.

3D printing is being explored as a manufacturing method for on-demand, personalized medicine.

Automated robotic arms manipulate nested trays and containers inside closed aseptic filling systems.

A twin-screw extruder can be used as a continuous wet granulator or as a continuous mixer for a wet gelatin mass.
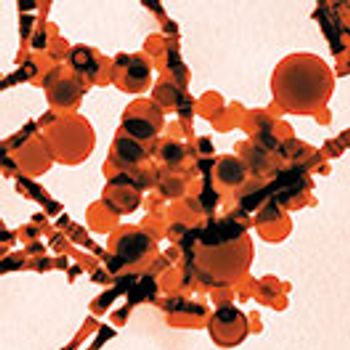
When choosing a test method to identify and measure particulate matter in inhaled drug products, the delivery device and drug form should be considered.

Modern technologies, including Industry 4.0 and the Industrial Internet of Things, offer opportunities to increase biopharmaceutical manufacturing efficiency.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates in inhaled drug products.

Automated robotic arms manipulate nested trays and containers inside closed aseptic filling systems.

Flow imaging microscopy can be used to identify particulates and their sources.

As more continuous processes move toward commercialization, users and equipment vendors are working to speed changeovers and address other needs.

The Parenteral Drug Association’s Data Integrity Task Force helps industry members understand regulatory requirements for data integrity.

The pharmaceutical industry is adopting Industry 4.0 and emerging technologies to improve product quality and manufacturing efficiency.

Pharma 4.0 envisions highly efficient automated processes, which could be continuous, batch, or a hybrid of these, driven by an integrated manufacturing control strategy.
Particle engineering using jet milling or spray drying can be used to obtain appropriate particle characteristics for inhalation drug products.

Process analytical technology and advanced process control strategies can be applied to either batch or continuous manufacturing of solid-dosage drugs.

Puncture and aerosolization tests measure the effectiveness of hard-shell capsules used in dry powder inhalers (DPIs) for inhaled drug products.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates.

Automation systems, vehicles, and robots improve efficiency of transporting materials and finished goods in pharmaceutical warehouses.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.

The Industrial Internet of Things and new tools such as smart glasses can improve training and daily tasks for pharmaceutical manufacturing operations.

The Industrial Internet of Things can be used in the bio/pharmaceutical industry to monitor equipment health, optimize processes, and enable modular facilities.