
As I visited the exhibits and attended the conference sessions at INTERPHEX 2013 last week, I noticed a focus on equipment flexibility and ease-of-cleaning and changeover that enables manufacturing efficiency.
Jennifer Markarian is manufacturing reporter for Pharmaceutical Technology.

As I visited the exhibits and attended the conference sessions at INTERPHEX 2013 last week, I noticed a focus on equipment flexibility and ease-of-cleaning and changeover that enables manufacturing efficiency.

The challenge of developing orally inhaled nasal drug products (OINDPs) is complicated by the interplay between drug-delivery devices and formulation.

Speakers discuss modularization, single-use systems, and process validation in a series of podcasts available on the Pharmaceutical Technology website.
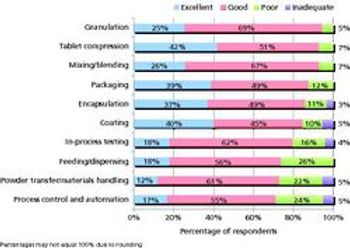
Pharmaceutical Technology's survey of manufacturing equipment and trends shows satisfaction with utility and innovation in most solid-dosage and parenteral drug-manufacturing equipment.

Pharmaceutical Technology will be moderating a panel discussion, “Lessons Learned: Successes and Challenges in Implementing Quality by Design,” on Wednesday Apr. 24 from 10:15 to 11:15 at INTERPHEX in New York City.

Pharmaceutical Technology interviewed some of the speakers for the upcoming INTERPHEX 2013 conference to get a “sneak peek” of their presentations.

A Pharmaceutical Technology survey shows satisfaction with utility and innovation in most solid dosage and parenteral drug-manufacturing equipment.

Industry is moving toward closed-loop control of continuous processing.

Representatives from Merck, Pfizer, and Novartis shared their recent efforts in applying quality-by-design (QbD) concepts to analytical methods, and Todd Cecil from USP explained the related new draft USP chapter in a symposium at Pittcon on Monday, March 18, 2013.

The New Jersey-licensed specialty pharmacy recalls magnesium sulfate products and halts all production operations.

Process analytical technology (PAT) is being successfully used to improve understanding and optimize pharmaceutical unit operations, but greater value can be obtained by integrating PAT with overall process control of a continuous manufacturing system. Pharmaceutical Technology spoke with Ivo Backx, manager of business and project development for the pharmaceutical industry at Siemens Industry Automation Division, to gain insight on the issues involved.

Product sold online in Malaysia and Indonesia infringes on the company’s trademark.

A GBI Research report says the opioid market will be driven by development of new products and post-marketing studies designed to reduce abuse risk.

Engineering groups at Rutgers and MIT constructed solid-dosage continuous manufacturing lines and transferred the technology to pharmaceutical industry partners.

Are equipment innovations keeping up with manufacturers’ needs? What do industry members think about quality by design and process analytical technology? The editors at Pharmaceutical Technology and Pharmaceutical Technology Europe are currently running a survey of trends in finished drug product manufacturing and innovation in pharmaceutical equipment and manufacturing to gain feedback on these and other questions.

Group sales were down, but R&D progress points to future growth.

In an article called “Beyond magic bullets: true innovation in health care,” published in the February 2013 issue of Nature Reviews Drug Discovery, scientists from Janssen encouraged the healthcare industry to think outside of the drug product-focused box and look at an integrated approach to care, said a Janssen press release.

Two general chapters on elemental impurities? limits and procedures are to become official Feb. 1, 2013, with implementation proposed for May 1, 2014.

Revisions to the United States Pharmacopeia (USP) General ChapterHeavy Metals have been much discussed over the past decade.

Rutgers engineers constructed a direct compaction line in collaboration with Janssen.

Recently, after reading about the severity of this year’s flu season, I finally went and got my vaccine, which my doctor had been out of when I tried in October.

Micronization can be performed with a jet mill or bead mill.

Eli Lilly expects overall revenue growth in the coming year.

Chemical engineers at the Massachusetts Institute of Technology (MIT) have designed an injectable gel with improved durability for use in drug delivery.

A University of Connecticut chemical engineering professor received a National Science Foundation grant to study how nanoparticles flow in the bloodstream, which is crucial for their use in drug delivery.

Plastic is finding increased use in vials and syringes as concerns about glass breakage and delamination and desire for increased functionality lead pharmaceutical companies to consider alternatives.

How to optimize continuous processing and make it work in pharmaceutical manufacturing is a crucial issue.

The US Supreme Court accepted an appeal by the Federal Trade Commission of a decision that upheld an arrangement of payments by Solvay Pharmaceuticals to generic drug companies to postpone introduction of generic versions of its branded testosterone-replacement drug.

The Polymer Processing Institute (PPI), an independent, not-for-profit, research and development organization that is headquartered at the New Jersey Institute of Technology (NJIT), has expanded its mission of working with industry and government to advance the field of polymer processing to include generating and using fundamental knowledge related to incorporating APIs into polymer excipients through hot-melt extrusion.

GlaxoSmithKline announced its intent to increase ownership in its publicly-listed, consumer-healthcare subsidiary in India and in GlaxoSmithKline Consumer Nigeria as part of its emerging-markets investment strategy.