
There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients.

There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients.

Quality-by-design submissions may reduce supplements and improve change management.

After two centuries, there's no reason to maintain two tablet compression tooling standards.

A. Nair discusses patent disputes in India.

If not properly monitored, filters and plastic bags can keep back more than they should.

New packaging options monitor and protect temperature-sensitive products.

The United States Pharmacopeia emphasizes mechanical calibration and a performance test to esnure integrity of the dissolution procedure.
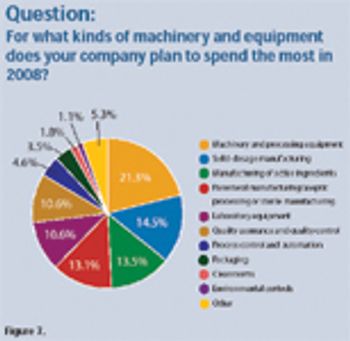
The pharmaceutical industry plans moderate increases in spending for equipment and machinery in 2008. Investments include equipment for solid-dosage manufacturing, active pharmaceutical ingredients, and parenteral manufacturing.
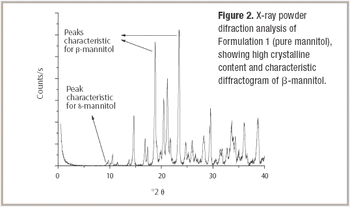
Mannitol is the most commonly used bulking agent in freeze-drying formulation design. The benefit of using mannitol is that it crystallizes during freezing and permits drying processes at higher product temperatures, and thus with higher sublimation rates relative to purely amorphous systems (1). Mannitol, however, is known to form different crystalline modifications which compromises reproducibility of product characteristics and storage stability due to phase transformations (2, 3).
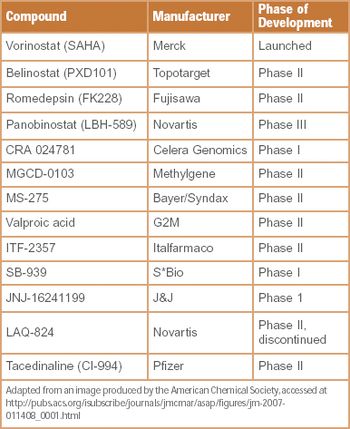
New research and ideas for March 2008

Contract manufacturers of APIs and intermediates are cautiously optimistic.

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.
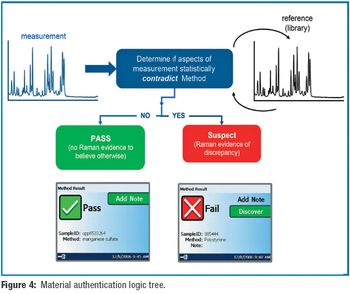
Using a handheld Raman spectrometer, the authors developed methods for 28 commonly used excipients and active ingredients.

SAFC is delivering on its plan for double-digit annual growth by increasing its businesses through organic growth and targeted technology acquisitions.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.

Editors' Picks of Pharmaceutical Science & Technology Innovations; Analytical system provides multiuser capability; Encapsulator aids dosage design; Versatile drive offers quick setup
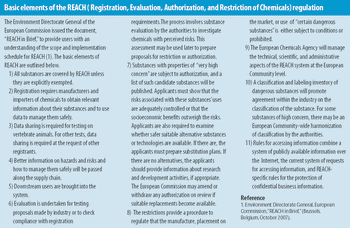
The European Union's REACH initiative has the potential to affect the flow of chemicals into the pharmaceutical suppy chain.

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.
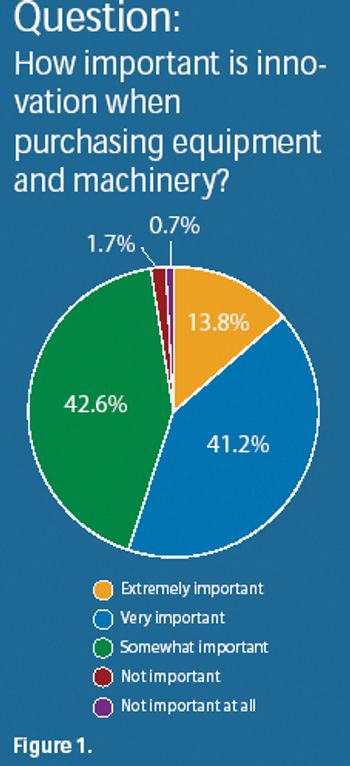
Results from Pharmaceutical Technology's Equipment and Machinery Trends survey and industry members provide insight into product innovation

CMOs have positive outlook for 2008 but are wary of competitive pressures.

A preview of some product enhancements and launches for Interphex 2008, the large trade show being held Mar. 26–28 in Philadelphia.
A news roundup for March 2008.

In this topical review, the authors discuss the rationale behind microstructural requirements for biopharmaceutical equipment and problems that may be encountered during the fabrication of high-performance corrosion-resistant equipment.

Brief pharmaceutical news items for March 2008.
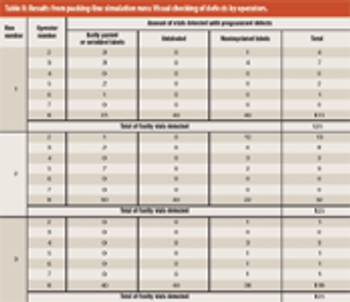
This article focuses on upgrading and improving a packing process to comply with current good manufacturing practices. The authors sought to maintain proper quality assurance for finished products.