
Bioprocessing and Sterile Manufacturing

Bioprocessing and Sterile Manufacturing
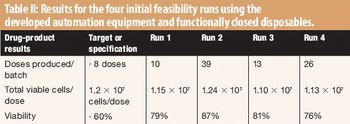
The authors developed automated equipment that uses functionally closed disposables to perform cellular and ribonucleic processing.

The authors discuss various approaches and related issues, including production of difficult-to express proteins using cell-free expression systems, scalability of protein expression, and site-specific chemical modifications.

The author reviews the state of downstream processing and considers potential solutions, including the streamlining of full processes and borrowed technologies.
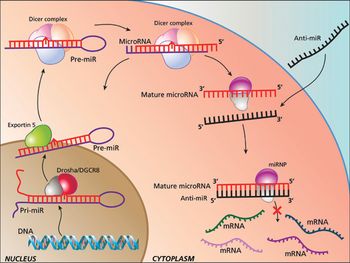
The authors provide further insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.
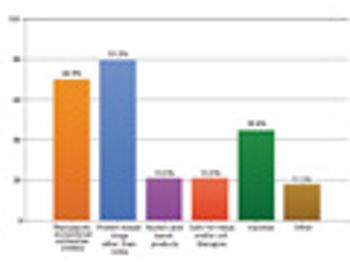
Results from our annual survey. This article contains online bonus material and is part of a special issue on Bioprocessing and Sterile Manufacturing.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

The author describes recent developments to help overcome the downstream processing bottleneck. This article is part of a special issue on Sterile Manufacturing and Bioprocessing.

Linking peptides to polyethylene glycol, or PEGylation, has helped improve pharmaceutical therapeutics in several ways. A wave of new techniques is now ushering in further advances.

A technical forum moderated by Patricia Van Arnum