
The updated edition of William Whyte's book provides information for novices and seasoned professionals alike.

The updated edition of William Whyte's book provides information for novices and seasoned professionals alike.
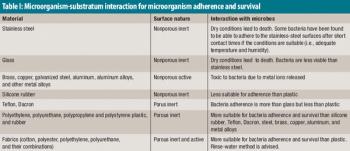
The author reviews test methods for microbiological cleaning processes and suggests ways to improve microbial bioburden method suitability studies.

Serialized product codes serve as the foundation of ePedigree records and product traceability.

Fallout escalates from McNeil recall and Genzyme shortages as regulators review oversight.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

The authors discuss the statistical tools used in experimental planning and stategy and how to evaluate the resulting design space and its graphical representation.

Cases of overlooking proper packaging, reconstitution, directions, and dissolution.

FDA chemistry reviewers in the Office of Generic Drugs provide an overview of common deficiences cited throughout the Chemistry, Manufacturing, and Controls section of ANDAs.

Manufacturers and regulators on both sides of the ocean move to ensure the safety of heparin and other globally distributed drug products.

The president of Jost Chemical talks about client demands, supplier audits, and more.

Industry can meet its responsibility to society by considering innovative pricing and partnerships.

Pharmaceutical Technology's annual analysis of the pharmaceutical majors' activities shows a shrinking global manufacturing network amidst company restructuring, product intensification in biologics, and a strategic focus and push to emerging markets.

The authors used common solvents to develop an initial solvent-screening method for laboratory-scale research to determine the solubility, polymorphism, and crystal properties of various active ingredients.

Relationship management is a limiting factor to growth in biomanufacturing outsourcing.

Editors' picks of pharmaceutical science and technology innovations.

Pharmaceutical Technology talked to experts in siRNA-drug development to gain insight into the characteristics, processes, and challenges of this class of therapeutics.

Chemocatalytic and biocatalytic approaches in asymmetric synthesis help provide a pathway for producing single-enantiomer drugs.

Green chemistry in pharmaceutical development often centers around approaches in the synthesis of the active ingredient, but green-chemistry techniques also can be applied to drug-product manufacturing, formulation development, and drug delivery.

Three therapies under review could help Americans attain a healthy weight.
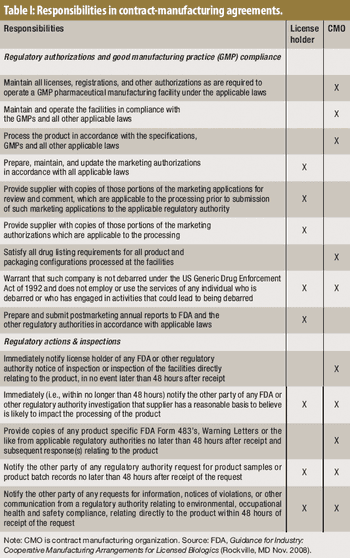
Before embarking on an outsourcing relationship, it's important to be aware of FDA's expectations.

Big Pharma?s Manufacturing Strategy: What is the Next Move?